Electrolyte, known as the blood of lithium-ion batteries, plays a decisive role in the main performance of lithium-ion batteries such as operating temperature, specific energy, cycle efficiency, and safety. Lithium hexafluorophosphate is the core material for the production of electrolyte, and has been commercialized as an electrolyte lithium salt. In the preparation process of lithium hexafluorophosphate electrolyte, a certain amount of hydrogen fluoride, water and other metal ions will inevitably remain, and the existence of metal ions will have a significant impact on the negative electrode material. The high concentration of metal impurity ions will not only lead to a decrease in the reversible specific capacity of lithium-ion batteries, but the precipitation of metal impurity ions may also lead to the inability to form an effective passivation layer on the surface of graphite electrodes and damage the entire battery. Therefore, the standard stipulates that lithium hexafluorophosphate The content limit of each metal element in the electrolyte.
The routine test on the computer after digestion generally dilutes the lithium hexafluorophosphate electrolyte in a large amount, which may make the actual content in the electrolyte low ; moreover, new impurities may also be introduced during the digestion process, which will affect the content of each element in the lithium hexafluorophosphate electrolyte. In addition, according to the standard "Lithium Hexafluorophosphate Electrolyte" (HG/T 4067-2015) , the lithium hexafluorophosphate electrolyte is directly injected after dilution to determine the content of each element without digestion process. Therefore, this application center dilutes the lithium hexafluorophosphate electrolyte with 20% ethanol, and uses an inductively coupled plasma optical emission spectrometer (ICP-OES), with TEC refrigeration accessories, a hydrofluoric acid-resistant sampling system, and an organic sampling system to test the two lithium hexafluorophosphate electrolytes. The accurate test of the element content is carried out, and the test data has good parallelism and standard addition recovery rate, which can be used as an analysis method for the actual sample of lithium hexafluorophosphate electrolyte.
Experimental part
Instrument
Table 1 Inductively coupled plasma spectrometer

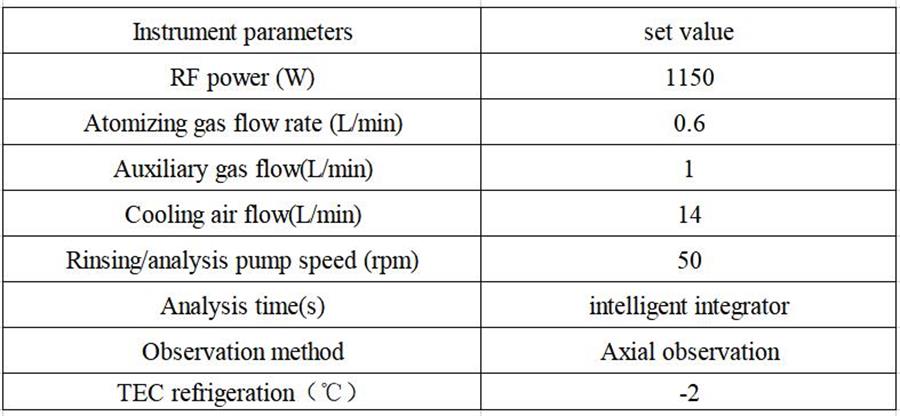
Table 2 Detection parameters of inductively coupled plasma spectrometer
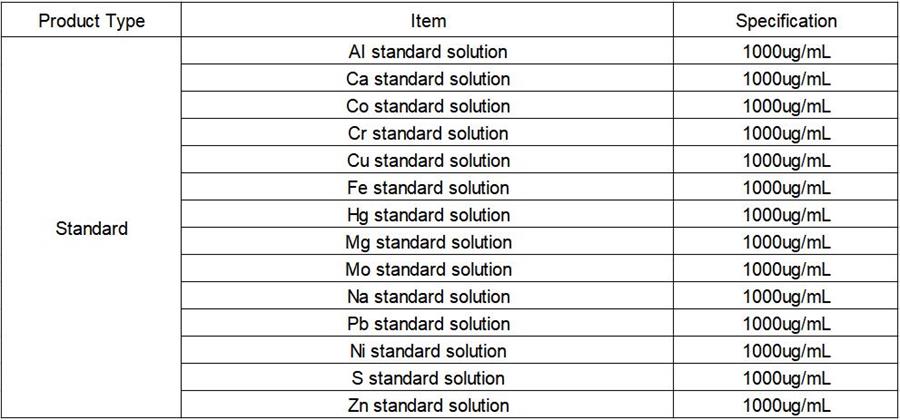
Reagents and Standards
- Reagent: electronic grade absolute ethanol;
- Pure water: 18.2 MΩ·cm deionized water;
- Standard solution: Al, Ca, Co, Cr, Cu, Fe, Hg, Mg, Mo, Ni, Na, Pb, S, Zn single element standard solution, 1000 ug/mL, National Institute of Nonferrous Metals.
Sample pretreatment
Weigh 2.0 g of lithium hexafluorophosphate electrolyte, and dilute to 10.0 g with 20% ethanol.
Standard curve and detection limit
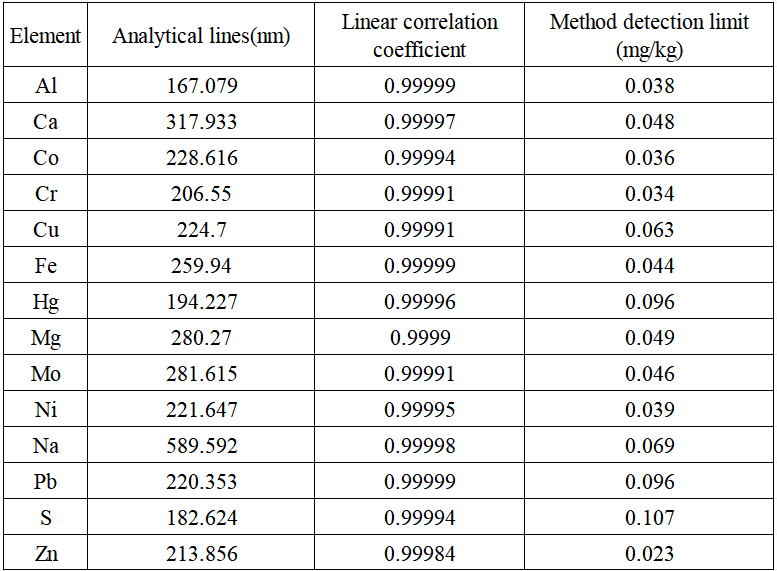
Select the appropriate analytical line of the analyte to draw a standard curve, and the test results show that the linear correlation coefficient of the analyte is greater than 0.9990. The sample concentration corresponding to 3 times the standard deviation of the measured value obtained by continuous analysis of the blank sample for 11 times was used as the detection limit of the instrument. See Table 3 for the linear correlation coefficients of the standard curves, analytical lines and detection limits of each analyte.
Table 3 Analytical lines, linear correlation coefficients and detection limits of analytes
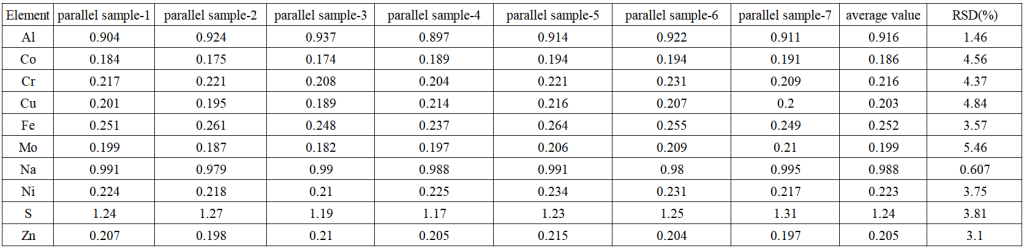
Method precision test
A second test was carried out on the samples prepared in parallel with 7 lithium hexafluorophosphate electrolyte samples spiked. The results showed that the RSD of each element was less than 5.0%, indicating that the precision of the method was good. The precision test results of each element method are shown in Table 4 below.
Table 4 Precision test results of lithium hexafluorophosphate electrolyte spiked samples (unit: mg/Kg)
Actual sample spike recovery test
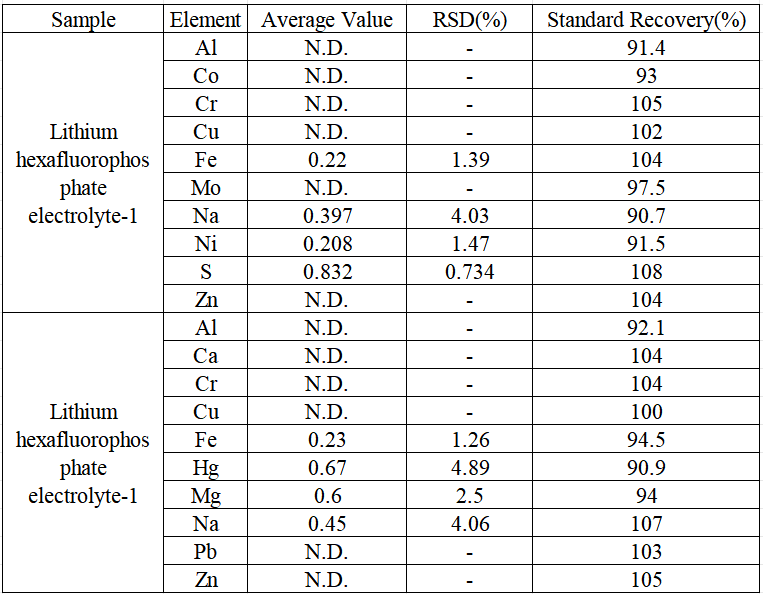
Two kinds of lithium hexafluorophosphate electrolyte samples were tested for recovery rate of standardization. Each sample was spiked at an appropriate concentration according to the element content. As shown in Table 6, the recovery rate of standardization for each sample was between 90.7% and 108%.
Table 5 Sample test results and spiked recoveries (unit: mg/kg)
Epilogue
In this experiment, 20% ethanol was used to dilute the lithium hexafluorophosphate electrolyte, and the lead, iron, copper, zinc, chromium, aluminum, sodium, calcium, magnesium, mercury, sulfur, cobalt, nickel, and molybdenum in the diluted sample were determined by ICP-OES. A method was established for the subsequent determination of the content of impurity elements in lithium hexafluorophosphate electrolyte. From the experimental results, the linear correlation coefficients of the established standard curves are all greater than 0.9990, the precision test RSDs of the elements to be measured in the actual samples are all less than 5.0%, and the recovery rates of each element to be measured in the actual samples are in the range of 90-108 %, the element detection limit range is 0.023 ~ 0.107 mg/kg; it shows that the sample test precision and accuracy are good, and this method can be applied to iron, sodium, magnesium, mercury, sulfur, nickel, molybdenum in lithium hexafluorophosphate electrolyte samples and other element content tests.
Appendix
Equipment and Consumables Solutions
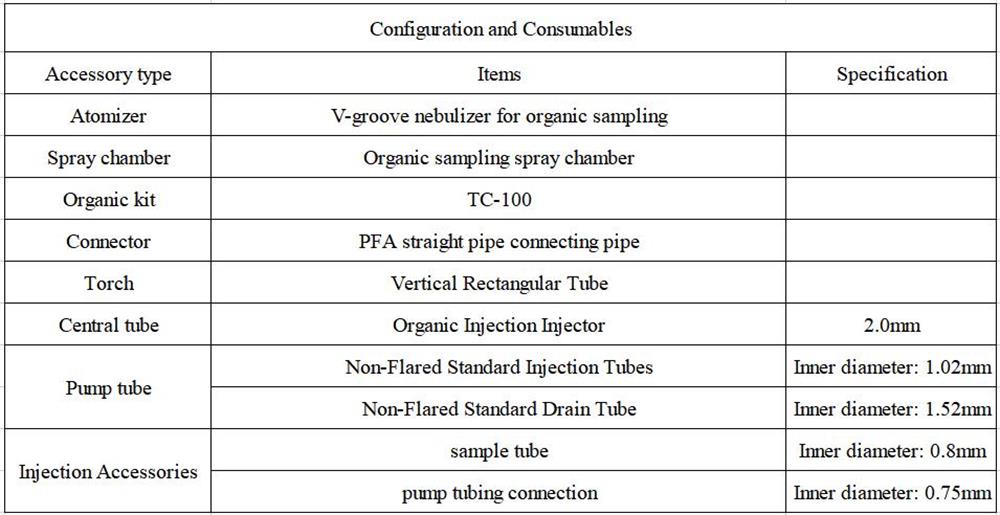
1.Configuration details of EXPEC 6500D organic sampling system
2. Reagents and Standards