Genotoxic impurities (or genotoxic impurities) refer to a class of substances that can directly or indirectly damage cellular DNA, produce genetic mutations or in vivo mutagenesis, and have the potential or tendency to cause cancer. In recent years, large-scale medical accidents have occurred due to the presence of trace amounts of genotoxic impurities in drugs already on the market, and subsequent drug recalls have occurred from time to time. FDA, EMA, and ICH have successively issued guidance on genotoxic impurities, recommending the use of the threshold of toxicological concern TTC (1.5 μg/day) as the acceptable limit for genotoxic impurities. The 2020 version of my country's "Chinese Pharmacopoeia" has added the "Guiding Principles for the Control of Genotoxic Impurities", and pharmaceutical companies are paying more and more attention to the control and detection of genotoxic impurities during the research and development process.
Sulfonate compounds, as a typical class of genotoxic impurities, can directly or indirectly metabolize activated alkylated DNA. Existing gas phase and gas phase detection methods for sulfonate ester compounds usually require derivatization, which is cumbersome to operate. Using GC for detection has poor qualitative capabilities and low sensitivity. Although the GC-MS method can improve detection sensitivity, it has poor anti-interference ability for some drugs with complex matrices. In comparison, the GC-MS/MS method has high anti-interference ability to deal with complex drug matrices, and has high detection sensitivity and good accuracy. It is an effective method for detecting sulfonate ester compounds in drugs. Therefore, this application center established a rapid and highly sensitive detection method for p-toluenesulfonate based on a self-developed GC-MS/MS platform for three genotoxic impurities of p-toluenesulfonate.
Keywords: GC-MS/MS, pharmaceuticals, oral pharmaceuticals, p-toluenesulfonate, genotoxic impurities
Experimental part
instrument
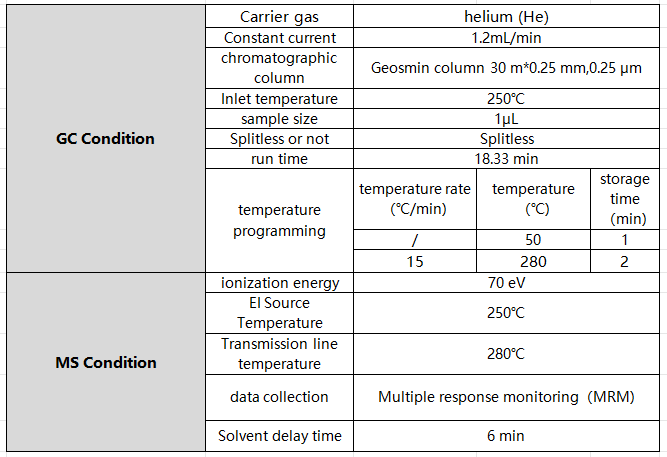
Table 1 Gas Chromatography -Triple Quadrupole Tandem Mass Spectrometer

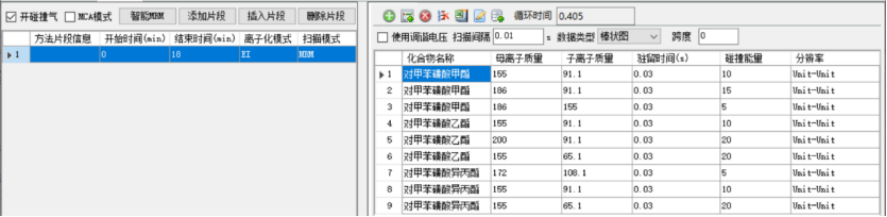
Table 2 Gas chromatography-triple quadrupole tandem mass spectrometer parameters
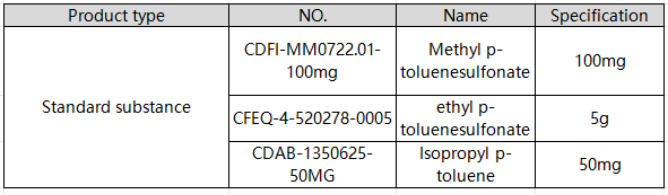
Reagents and standards
Standard products: Methyl p-toluenesulfonate, ethyl p-toluenesulfonate, and isopropyl p-toluenesulfonate were purchased from Stanford Analytical Chemicals and stored in a refrigerator at 4°C.
Reagents: Acetone and ethyl acetate are both chromatography grade.
Sample preparation
(1) Water-soluble drugs
Weigh the drug powder, add water and shake evenly, ultrasonic for 10 minutes, then add ethyl acetate, shake and mix thoroughly, then fully extract by ultrasonic, take the upper organic phase, remove water with anhydrous sodium sulfate, filter the membrane, and run the test on the machine.
(2) Fat-soluble drugs
Weigh the drug powder, add ethyl acetate, shake evenly, sonicate for 10 minutes, filter the membrane, and run the test on the machine.
Linearity and detection limits
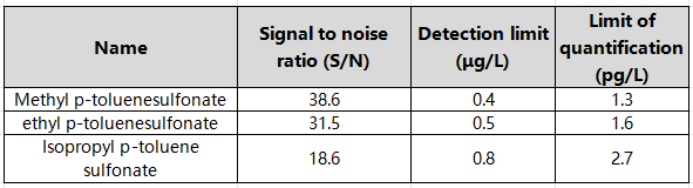
Within the corresponding linear range, the linear coefficients r of the three p-toluenesulfonate compounds are all above 0.995, indicating good linearity. Inject the sample with 5 μg/L standard solution, and calculate the limit of quantification and detection limit according to S/N=10 and S/N=3. The detection limit is 0.4~0.8 μg/L, and the limit of quantitation is 1.3~2.7 μg/L. , the specific results are shown in Table 3. The total ion chromatogram of p-toluenesulfonate compounds is shown in Figure 1.
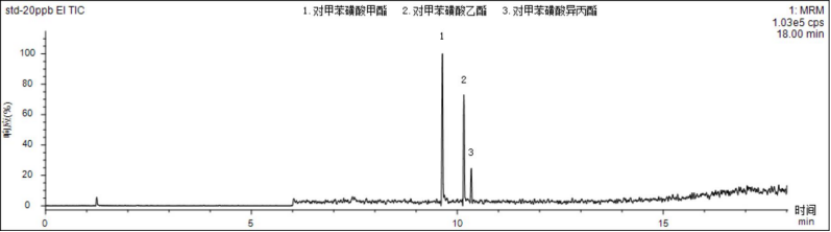
Figure 1 Total ion chromatogram of 20.0 μg/L p-toluenesulfonate compounds
Table 3 Signal-to-noise ratio of each component of p-toluenesulfonate (at 5 μg/L concentration level) and detection limit and quantitation limit
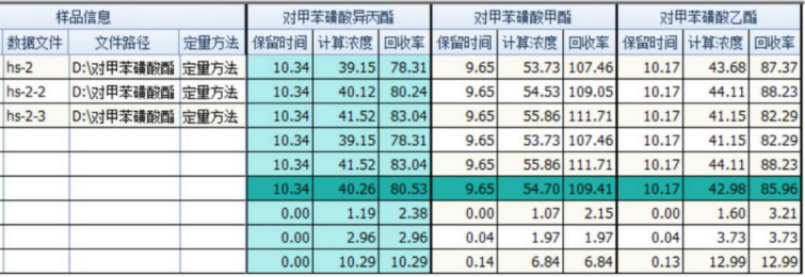
Spiked recovery rate
The blank drug was tested for spiked recovery rate. The spiked concentration was 1 μg/g. Three consecutive injections were made. The calculated concentration (unit: μg/L) and recovery rate of each target compound were as follows. Three kinds of p-toluene sulfonate The average recovery rate of acid esters is between 80% and 110%.
test sample
The actual drugs were processed according to the sample pretreatment method in 2.3. None of the three p-toluenesulfonate esters were detected in the actual drugs. The chromatogram is shown in Figure 2.
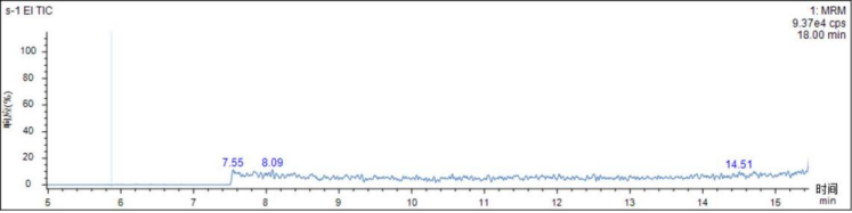
Figure 2 Actual drug chromatogram
Conclusion
This article established an analytical method for the determination of three p-toluenesulfonate compounds in pharmaceuticals using a triple quadrupole GC-MS. This article examines the linearity, precision, sensitivity and accuracy of the method. The results show that the standard curve is linear, the correlation coefficient is greater than 0.995, the method precision is less than 10%, the detection limit is less than 1 μg/L, and the quantitation limit is low. At 3 μg/L, the recovery rate is between 80% and 110%. Therefore, this method can perform rapid, accurate and sensitive quantitative analysis of three p-toluenesulfonate compounds in pharmaceuticals.
l Appendix
Equipment and consumables solutions
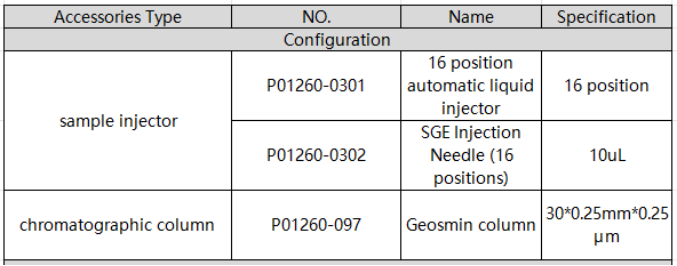
1. EXPEC 5231 system standard configuration details
2. Standard products