Compared with traditional batteries, lithium-ion batteries have attracted much attention due to their high energy density, long service life and no memory effect . As an important part of lithium batteries, cathode materials have a direct impact on the overall performance of lithium batteries. Traditional cathode materials include lithium cobaltate, lithium manganate, lithium nickelate, and lithium ferrite, among which lithium cobaltate has high cost, lithium nickelate has poor stability, lithium manganate has low capacity, lithium ferrite has low discharge voltage and low tap density . In order to solve the above problems, researchers have developed nickel-cobalt-manganese ternary materials . In ternary materials, Ni is beneficial to improve the reversible lithium intercalation capacity of the material; Co can make it easier to deintercalate Li+ and improve the electrical conductivity of the material; Mn can not only reduce the cost of the material, but also provide a stable framework for the material and improve the electrical conductivity of the material. The structural stability of the material. In order to optimize the performance of ternary materials, it is necessary to strictly control the content of each metal element in the material.
There are 5 current standards for the determination of element content in nickel-cobalt-manganese materials, among which inductively coupled plasma optical emission spectrometry (ICP-OES) is the main method, and a small part uses chemical methods to determine the corresponding metal content. Compared with the chemical method, the ICP-OES method has many advantages such as simple operation, wide linear range, low detection limit, and simultaneous determination of multi-element content. The substances measured in the relevant standards are all substances with high purity, but the mixture of nickel-cobalt-manganese ternary materials and other substances may be encountered in the actual process of production, and the samples discussed in this paper contain a large amount of carbon black. The conventional electric hot plate digestion method It is difficult to completely digest the carbon, and the effect of leaching is also poor. However, because the argon gas is introduced into the reaction chamber by the super microwave, the boiling point of the acid is forcibly increased, so that the digestion effect of the sample is better . Therefore, the traditional hydrochloric acid digestion method may not be effective in digesting the intermediate products of the actual process. Therefore, the sample was soaked in nitric acid-hydrochloric acid mixed acid and then digested with a super microwave digestion instrument, and then filtered to test the content of each element in the digestion solution.
Referring to the above standard method, the application center adopts the mixed acid digestion system of nitric acid-hydrochloric acid, uses the super microwave chemical workstation to digest the sample, filters to obtain the filtrate, and then uses the inductively coupled plasma emission spectrometry The contents of six elements, nickel, cobalt, manganese, aluminum, copper and lithium, are accurately determined.
Key words: super microwave, lithium batteries, ternary materials, ICP-OES, impurity elements
Experimental part
Instrument
Table 1 Instrument


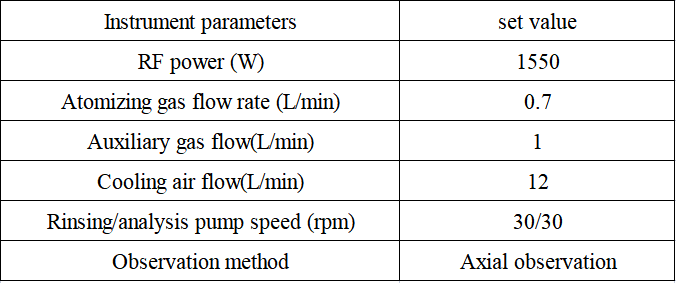
Table 2 Detection parameters of inductively coupled plasma emission spectrometer
Reagents and Standards
Reagent: premium pure nitric acid;
Pure water: 18.2 MΩ·cm deionized water (25°C);
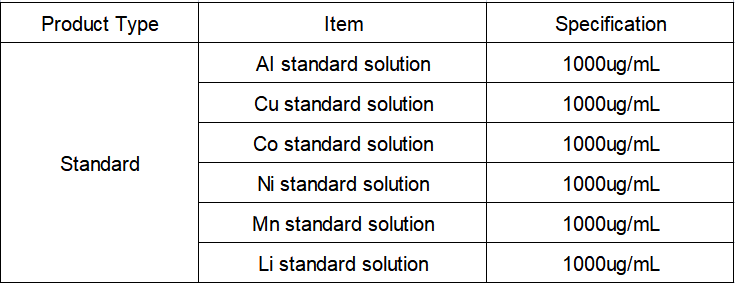
Standard solution: Al, Li, Mn, Cu, Li, Ni-single element standard solution, 1000 μg/mL, National Institute of Nonferrous Metals.
Reagents and Standards
Reagent: premium pure nitric acid;
Pure water: 18.2 MΩ·cm deionized water (25°C);
Standard solution: Al, Li, Mn, Cu, Li, Ni-single element standard solution, 1000 μg/mL, National Institute of Nonferrous Metals.
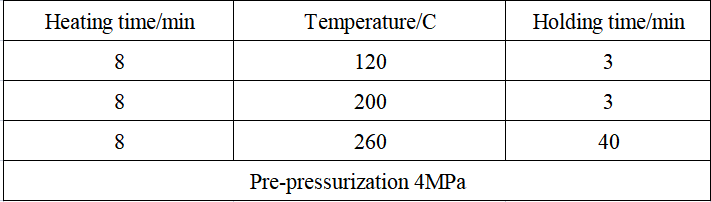
Table 3 Super microwave heating digestion program
Sample Status
Table 4 The state of the actual sample before and after digestion

Test Results
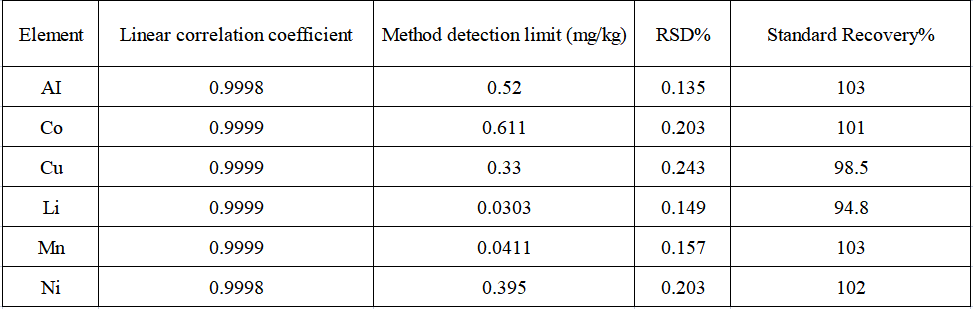
The linear coefficients of each element are greater than 0.9998. Repeat the blank experiment 11 times, convert each measurement result into the concentration in the sample, calculate 3 times the standard deviation of 11 parallel determinations and multiply it by the dilution factor to obtain the corresponding method detection limit. Seven parallel samples were prepared for the precision test of the method, and the RSD of each element's internal precision was less than 0.3%. The recoveries of each element in the actual samples were 94.8%~103%.
Table 5 Test results
Actual Sample Test
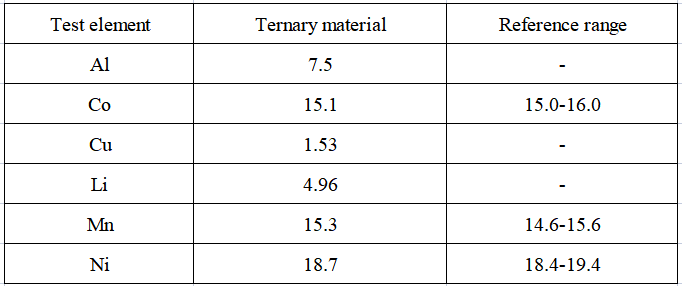
The test results of the content of each element in the actual sample are shown in Table 5. The contents of the three elements of nickel, cobalt, and manganese are all within the reference range, indicating that this method can be effectively applied to the main elements in nickel, cobalt, and manganese ternary materials such as nickel, cobalt, manganese, and aluminum. , copper, lithium content test.
Table 5 Test results of the content of analytes in actual samples (unit: %)
Epilogue
The linear coefficient of the standard curve of each analyte in the sample is 1.00000, the internal precision test RSD of the analyte in the actual sample is less than 0.3%, and the recovery rate of each analyte in the actual sample is in the range of 94.8% ~ 103% , the element detection limit range is 0.03 ~ 0.61 mg/kg, indicating that the precision and accuracy of the sample test are good, and this method can be effectively applied to aluminum, cobalt, copper, lithium, manganese, nickel6 Determination of element content.
Appendix
Equipment and Consumables Solutions
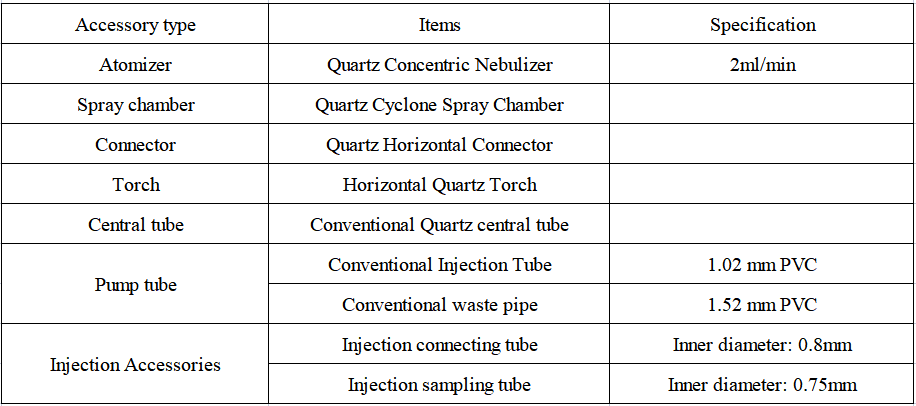
1. Configuration details of EXPEC 6500 ICP-OES standard sampling system
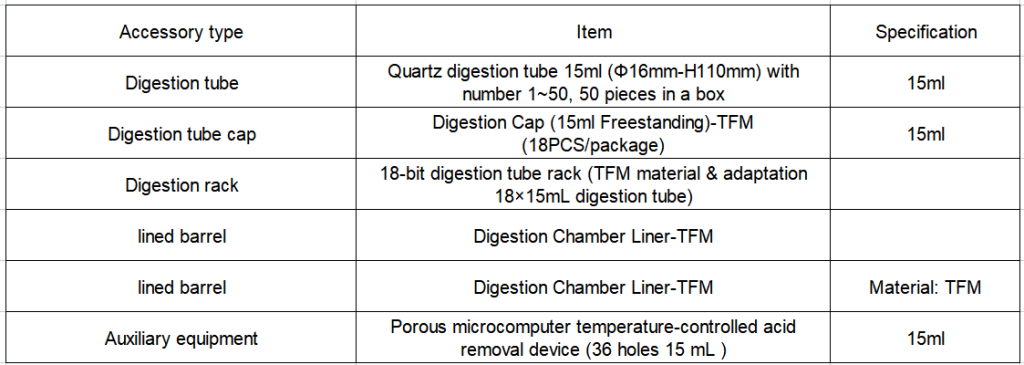
2. Configuration details of EXPEC 790S Super Microwave Chemical Workstation
3. Reagents and Standards