
Introduction
Odor in water bodies is not only a sensory indicator for evaluating water environmental quality, but also a direct reflection of overall water quality. Water odor is primarily caused by the presence of odor-causing compounds whose concentrations exceed their human odor threshold values (Table 1). Common odor-causing substances in aquatic environments include sulfur-containing compounds with putrid or sulfurous odors, as well as earthy-musty compounds such as geosmin (GSM) and 2-methylisoborneol (2-MIB).
These odor-causing compounds typically exhibit extremely low odor threshold values, generally at the ng/L (10⁻⁹) concentration level. For example, the odor threshold values of geosmin and 2-methylisoborneol are approximately 10 ng/L, while those of dimethyl disulfide and dimethyl trisulfide are around 30 ng/L. The combination of ultra-low concentration levels and the complex composition of water matrices pose significant analytical challenges for the determination of trace odor-causing compounds in water environments.
Table 1. Typical Odor-Causing Compounds and Their Odor Characteristics
| Odor Type | Typical Odor-Causing Compounds |
| Earthy / Musty odor | 2-Methylisoborneol (2-MIB), Geosmin (GSM), 2,4,6-Trichloroanisole |
| Putrid odor | Sulfides, thiols, and other sulfur-containing organic compounds |
| Fishy / Grassy odor | Hexanal, 2-Octenal, Nonanal, 2,6-Nonadienal, 2,4-Decadienal, Heptanal, 2,4-Heptadienal, Benzaldehyde, β-Cyclocitral, etc. |
| Chemical odor | Bis(2-chloro-1-methylethyl) ether, 2-Ethyl-4-methyl-1,3-dioxolane, 2,5,5-Trimethyl-1,3-dioxane, 2-Ethyl-5,5-dimethyl-1,3-dioxane, etc. |
In 2016, the International Organization for Standardization (ISO) issued ISO 17943:2016, which specifies the determination of 63 odor-causing compounds, including 2-methylisoborneol (2-MIB) and geosmin (GSM) in water using solid-phase microextraction (SPME). In the same year, China released the national standard GB/T 32470-2016, “Examination Methods for Odor Compounds in Drinking Water—Geosmin and 2-Methylisoborneol,” which also adopts SPME combined with gas chromatography–mass spectrometry (GC-MS) for the qualitative and quantitative analysis of 2-MIB and GSM in water. This method was subsequently incorporated in 2023 into GB/T 5750.8-2023, “Standard Examination Methods for Drinking Water—Part 8: Organic Parameters.”
The combination of solid-phase microextraction (SPME) and gas chromatography–mass spectrometry (GC-MS) integrates the efficient enrichment capability of SPME for odor-causing compounds with the powerful qualitative and quantitative performance of GC-MS. This integrated approach effectively overcomes sensitivity limitations commonly encountered in the analysis of trace-level odor compounds in water, while enabling accurate quantification of target analytes and reliable identification of unknown compounds. As such, SPME-GC-MS represents an important technical tool for early warning and assessment of water environmental pollution, and is particularly well suited for the determination of trace odor-causing substances in water.
In this study, referring to GB/T 5750.8-2023 and ISO 17943:2016, an analytical method for the determination of eight odor-causing compounds in water was established using the EXPEC 218 automated multifunctional sampling system (with integrated SPME sample preparation) coupled with the EXPEC 3750 gas chromatography–mass spectrometry system. The results demonstrate good linearity for all eight analytes over the concentration range of 0–200 ng/L, with correlation coefficients (R²) ranging from 0.9965 to 0.9995. The method precision ranged from 1.73% to 6.84%, and the spiked recovery values were between 97.85% and 112.90%. When a sample volume of 10 mL was used, the method detection limits (MDLs) for the analytes ranged from 1.01 ng/L to 5.97 ng/L. These performance parameters meet the requirements of relevant standards and minimum detection limits, providing strong technical support for the qualitative and quantitative determination of eight odor-causing compounds in drinking water and water environments.
Keywords:
Solid-phase microextraction (SPME); Gas chromatography–mass spectrometry (GC–MS); Odor-causing compounds; Drinking water; Trace analysis; Geosmin; 2-Methylisoborneol
Instruments and Reagents
Instruments:EXPEC 3750 Gas Chromatography–Mass Spectrometry (GC–MS) System ;EXPEC 218 Automated Multifunctional Sampling System (EXPEC Technology)

Figure 1. EXPEC 218 automated multifunctional sampling system coupled with the EXPEC 3750 gas chromatography–mass spectrometry system
Reagents and Materials
A mixed standard solution of eight odor-causing compounds (100 mg/L in methanol, ANPEL, Shanghai, China); 2-isobutyl-3-methoxypyrazine standard solution (100 mg/L in methanol, ANPEL, Shanghai, China); 1,2-dichlorobenzene-D₄ (100 mg/L, ANPEL); methanol (chromatographic grade); sodium chloride (analytical grade); and ultrapure water.
High-purity helium (purity ≥99.999%); 20 mL headspace vials; micropipettes (10 mL and 1 mL); and microsyringes (10 μL and 50 μL).
SPME fiber: DVB/CAR/PDMS (50 μm/30 μm, Supelco).
GC column: Rtx-624 (30 m × 0.25 mm × 1.4 μm).
Table 2. Basic Information of Odor-Causing Compounds
| No. | Compound | CAS NO. | Abbreviation | Quantifier Ion (m/z) | Qualifier Ion (m/z) |
| 1 | Dimethyl disulfide | 624-92-0 | DMDS | 94 | 79,45 |
| 2 | 2-Ethyl-5,5-dimethyl-1,3-dioxane | 4359-46-0 | 2-EMD | 87 | 43,59 |
| 3 | 2-Ethyl-5,5-dimethyl-1,3-dioxane | 768-58-1 | 2-EDD | 115 | 56,69 |
| 4 | Diethyl disulfide | 110-81-6 | DEDS | 122 | 94,79 |
| 5 | Dimethyl trisulfide | 3658-80-8 | DMTS | 126 | 45,79 |
| IS1 | 1,2-Dichlorobenzene-D₄ | 2199-69-1 | IMBP | 150 | 152,78 |
| 6 | Bis(2-chloro-1-methylethyl) ether | 108-60-1 | DCIP | 121 | 79,51 |
| IS2 | 2-Isobutyl-3- methoxypyrazine | 24683-00-9 | IBMP | 124 | 151,94 |
| 7 | 2-Methylisoborneol | 2371-42-8 | 2-MIB | 95 | 107,135 |
| 8 | Geosmin | 19700-21-1 | GSM | 112 | 125,97 |
Experimental Method
Preparation of Standard Solutions: Using methanol as the solvent, the mixed standard solution of eight odor-causing compounds was diluted to prepare an intermediate working solution with a concentration of 50 μg/L. Similarly, the internal standards 2-isobutyl-3-methoxypyrazine and 1,2-dichlorobenzene-D₄ were prepared as intermediate working solutions at a concentration of 50 μg/L in methanol.
Preparation of Calibration Standards: Fifteen-milliliter sample vials were used for calibration. Sodium chloride (3.5 g) and a magnetic stir bar were added to each vial, followed by 10 mL of ultrapure water. Appropriate volumes of the intermediate working solutions of the eight odor-causing compounds and the two internal standards were then added to obtain calibration standards with analyte concentrations of 0, 5, 10, 20, 50, 80, 100, 150, and 200 ng/L, while the internal standard concentration was maintained at 50 ng/L. The vials were sealed and prepared for analysis.
Preparation of Spiked Samples: Fifteen-milliliter sample vials were prepared by adding 3.5 g of sodium chloride, a magnetic stir bar, and 10 mL of tap water. Aliquots of the 50 μg/L intermediate working solutions of the eight odor-causing compounds and the two internal standards were added to obtain spiked samples with a final volume of 10 mL, a salt concentration of 35% (w/v), an internal standard concentration of 50 ng/L, and target analyte concentrations of 100 ng/L and 200 ng/L, respectively. The vials were sealed and subjected to analysis.
Results and Discussion
Based on the instrumental parameters listed in Table 3, total ion chromatograms (TICs) of the calibration standards were acquired. As shown in Figure 2, satisfactory separation was achieved for the eight odor-causing compounds and the internal standards, with all components exhibiting baseline separation.
Table 3. Instrumental Operating Conditions
| Instrument | Component | Parameter |
| SPME | Extraction temperature | 55℃ |
| Extraction time | 40 min | |
| Fiber type | 50 μm/30 μm (DVB/CAR/PDMS) | |
| Extraction depth | 13 mm (varies with vial specifications; optimization required) | |
| Desorption depth | 37 mm | |
| Desorption temperature | 240 ℃ | |
| Desorption time | 7 min | |
| Ionic strength | Sodium chloride: 3.5 g (35%, w/v) | |
| GC-MS | Injector temperature | 240℃ |
| Injection mode | Splitless | |
| Column | Rtx-624(30 m×0.25 mm×1.4 μm) | |
| Carrier gas flow rate | 1 mL/min | |
| Oven temperature program | 40 ℃,(hold 2 min), ramp at 10 °C/min to 230 °C, hold 10 min | |
| GC–MS interface temperature | 240 ℃ | |
| Ion source temperature | 240 ℃ | |
| Ionization energy | 70 eV | |
| Acquisition mode | SIM (quantifier and qualifier ions listed in Table 2) |
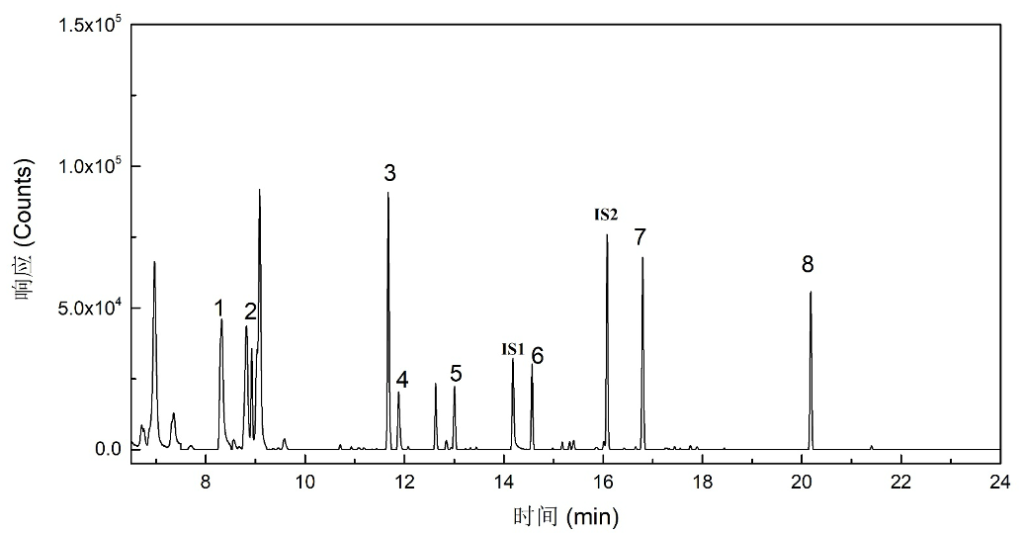
Figure 2. Selected ion chromatograms (SIM) of eight odor-causing compounds and two internal standards
1 – Dimethyl disulfide; 2 – 2-Ethyl-4-methyl-1,3-dioxolane; 3 – 2-Ethyl-5,5-dimethyl-1,3-dioxane; 4 – Diethyl disulfide; 5 – Dimethyl trisulfide; IS1 – 1,2-Dichlorobenzene-D₄; 6 – Bis(2-chloro-1-methylethyl) ether; IS2 – 2-Isobutyl-3-methoxypyrazine; 7 – 2-Methylisoborneol; 8 – Geosmin.
Calibration Curves
Based on the reference standards and instrumental conditions listed in Table 3, quantification was performed using the internal standard method. Good linearity was obtained for the eight odor-causing compounds over the concentration range of 0–200 ng/L, with correlation coefficients (R²) ranging from 0.9965 to 0.9995 (Table 4). The linearity fully meets the requirements for standard analytical applications. Representative calibration curves for selected analytes are shown below.
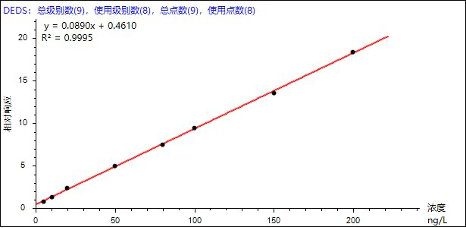
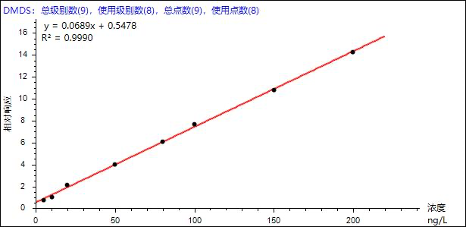
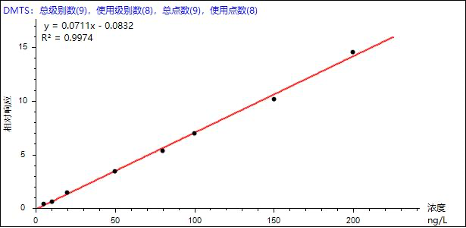
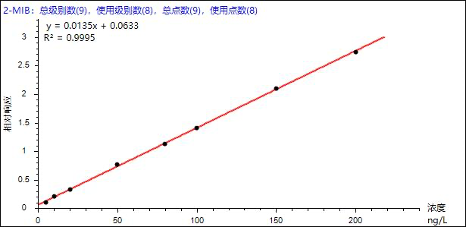
Figure 3. Representative calibration curves of selected analytes
Method Precision
Under the instrumental conditions and experimental procedures described in Table 3, six replicate measurements were performed for samples with a concentration of 150 ng/L. The relative standard deviation (RSD) was calculated to evaluate method precision, and the detailed results are summarized in Table 4. The precision values for the eight odor-causing compounds ranged from 1.73% to 6.84%, demonstrating good repeatability of the proposed method.
Table 4. Results of precision evaluation (concentration unit: ng/L)
| Compounds | Abbreviation | R2 | 1 | 2 | 3 | 4 | 5 | 6 | RSD(%) |
| Dimethyl disulfide | DMDS | 0.9990 | 141.36 | 154.84 | 151.48 | 149.67 | 159.54 | 144.25 | 4.45 |
| 2-Ethyl-4-methyl-1,3-dioxolane | 2-EMD | 0.9986 | 158.12 | 174.28 | 188.88 | 180.94 | 190.24 | 170.65 | 6.84 |
| 2-Ethyl-5,5-dimethyl-1,3-dioxane | 2-EDD | 0.9985 | 156.61 | 161.77 | 177.08 | 168.47 | 188.01 | 169.57 | 6.55 |
| Diethyl disulfide | DEDS | 0.9995 | 137.01 | 137.58 | 142.91 | 143.52 | 142.20 | 140.11 | 1.97 |
| Dimethyl trisulfide | DMTS | 0.9974 | 128.03 | 130.88 | 138.12 | 130.93 | 130.78 | 135.18 | 2.76 |
| Bis(2-chloro-1-methylethyl) ether | DCIP | 0.9965 | 125.83 | 124.08 | 119.23 | 120.30 | 124.18 | 119.16 | 2.38 |
| 2-Methylisoborneol | 2-MIB | 0.9995 | 128.82 | 130.08 | 129.32 | 124.03 | 127.85 | 125.31 | 1.87 |
| Geosmin | GSM | 0.9990 | 116.28 | 112.09 | 111.92 | 111.56 | 111.85 | 114.81 | 1.73 |
Method Detection Limits
Seven replicate samples with a concentration of 10 ng/L were prepared and analyzed under the instrumental conditions described above. The standard deviation (S) of the measured results was calculated. The method detection limit (MDL) was then determined according to the equation:
MDL = 3.143 × S (99% confidence level)
The calculated MDLs for the eight odor-causing compounds ranged from 1.01 ng/L to 5.97 ng/L, as summarized in Table 5, meeting the requirements of relevant standard analytical methods.
Table 5. Results of method detection limit calculation (concentration unit: ng/L)
| Compounds | Time(min) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | MDL |
| DMDS | 8.283 | 12.63 | 11.71 | 6.72 | 10.75 | 11.20 | 9.99 | 9.69 | 5.97 |
| 2-EMD | 8.810 | 13.49 | 9.86 | 12.21 | 11.75 | 11.23 | 10.97 | 10.27 | 3.86 |
| 2-EDD | 11.673 | 9.95 | 9.31 | 9.65 | 9.54 | 9.50 | 8.94 | 9.26 | 1.01 |
| DEDS | 11.880 | 10.57 | 10.19 | 11.42 | 9.36 | 9.64 | 9.65 | 9.31 | 2.40 |
| DMTS | 13.010 | 9.86 | 8.84 | 6.74 | 8.53 | 8.26 | 8.42 | 7.08 | 3.32 |
| DCIP | 14.576 | 8.03 | 8.81 | 8.36 | 8.28 | 9.32 | 10.10 | 9.12 | 2.26 |
| 2-MIB | 16.805 | 10.64 | 12.38 | 11.45 | 10.90 | 10.90 | 11.27 | 11.89 | 1.93 |
| GSM | 20.187 | 7.74 | 8.01 | 7.14 | 7.24 | 6.86 | 7.50 | 7.59 | 1.22 |
Sample Recovery
Under the instrumental conditions and experimental procedures described in Table 3, spiked samples were analyzed and the spike recovery was calculated. The recovery results are summarized in Table 6. The results show that, for spiking levels of 100 ng/L and 200 ng/L, the recoveries of all target compounds ranged from 97.85% to 112.90%, indicating good accuracy of the proposed method.
Table 6. Results of spike recovery experiments
| Basic Information | 100 ng/L | 200 ng/L | |||
| Compunds | Abbre. | R2 | Time(min) | Recovery(%) | Recovery(%) |
| Dimethyl disulfide | DMDS | 0.9990 | 8.283 | 109.55 | 102.80 |
| 2-Ethyl-4-methyl-1,3-dioxolane | 2-EMD | 0.9986 | 8.810 | 109.35 | 97.85 |
| 2-Ethyl-5,5-dimethyl-1,3-dioxane | 2-EDD | 0.9985 | 11.673 | 106.14 | 99.24 |
| Diethyl disulfide | DEDS | 0.9995 | 11.880 | 112.90 | 106.72 |
| Dimethyl trisulfide | DMTS | 0.9974 | 13.010 | 100.00 | 103.12 |
| 1,2-Dichlorobenzene-D₄ (IS1) | IMBP | - | 14.187 | - | - |
| Bis(2-chloro-1-methylethyl) ether | DCIP | 0.9965 | 14.576 | 109.59 | 98.87 |
| 2-Isobutyl-3-methoxypyrazine (IS2) | IBMP | - | 16.088 | - | - |
| 2-Methylisoborneol | 2-MIB | 0.9995 | 16.805 | 108.75 | 103.62 |
| Geosmin | GSM | 0.9990 | 20.187 | 104.98 | 99.67 |
Conclusion
A method for the determination of eight odor-causing compounds in water was established using the EXPEC 218 automated multifunctional sampling system (with integrated solid-phase microextraction) coupled with the EXPEC 3750 GC–MS system. Good analytical performance was achieved for all eight compounds, with correlation coefficients (R²) ranging from 0.9965 to 0.9995, method precision between 1.73% and 6.84%, and spike recoveries from 97.85% to 112.90%.
When a sample volume of 10 mL was used, the method detection limits (MDLs) ranged from 1.01 ng/L to 5.97 ng/L. The proposed method meets the requirements of relevant standards with respect to regulatory limits and minimum detection concentrations, and provides reliable technical support for the qualitative and quantitative analysis of eight odor-causing compounds in drinking water and water environments.
The analytical performance of the method is summarized as follows (overall results are listed in Table 7):
1. Correlation coefficients (R) for the eight odor-causing compounds ranged from 0.9965 to 0.9997;
2. Method precision ranged from 1.73% to 6.84%;
3. Method detection limits ranged from 1.01 ng/L to 5.97 ng/L;
4. Spike recoveries for all eight compounds ranged from 97.85% to 112.90%.
Table 7. Summary of method performance evaluation
| Compoudns | Abbre. | Internal Standard | Time(min) | R2 | RSD (%) | MDL (ng/L) |
| Dimethyl disulfide | DMDS | 1,2-Dichlorobenzene-D₄ | 8.283 | 0.9990 | 4.45 | 5.97 |
| 2-Ethyl-4-methyl-1,3-dioxolane | 2-EMD | 1,2-Dichlorobenzene-D₄ | 8.810 | 0.9986 | 6.84 | 3.86 |
| 2-Ethyl-5,5-dimethyl-1,3-dioxane | 2-EDD | 1,2-Dichlorobenzene-D₄ | 11.673 | 0.9985 | 6.55 | 1.01 |
| Diethyl disulfide | DEDS | 1,2-Dichlorobenzene-D₄ | 11.880 | 0.9995 | 1.97 | 2.40 |
| Dimethyl trisulfide | DMTS | 1,2-Dichlorobenzene-D₄ | 13.010 | 0.9974 | 2.76 | 3.32 |
| 1,2-Dichlorobenzene-D₄ (IS1) | IMBP | Internal standard 1 | 14.187 | - | - | - |
| Bis(2-chloro-1-methylethyl) ether | DCIP | 2-Isobutyl-3-methoxypyrazine | 14.576 | 0.9965 | 2.38 | 2.26 |
| 2-Isobutyl-3-methoxypyrazine (IS2) | IBMP | Internal standard 2 | 16.088 | - | - | - |
| 2-Methylisoborneol | 2-MIB | 2-Isobutyl-3-methoxypyrazine | 14.576 | 0.9995 | 1.87 | 1.93 |
| Geosmin | GSM | 2-Isobutyl-3-methoxypyrazine | 14.576 | 0.9990 | 1.73 | 1.22 |

