
Introduction
Phthalates (PAEs), also known as phthalic acid esters, are widely used in the manufacturing of plastic products as common plasticizers. They significantly improve the flexibility and processability of plastics. However, these compounds are classified as environmental hormones, and once they enter the human body, they can disrupt the endocrine system, posing potential health risks. According to the International Agency for Research on Cancer (IARC) under the World Health Organization (WHO), Di(2-ethylhexyl) phthalate (DEHP) has been listed as a Group 2B possible human carcinogen.
In June 2011, China’s National Health Commission (NHC) issued Announcement No.16, adding phthalates to the List of Non-Edible Substances and Easily Abused Food Additives That May Be Illegally Added to Food (hereinafter referred to as “the List”), explicitly prohibiting their direct use in food production. Nevertheless, due to their excellent plasticizing performance, certain phthalates are permitted within regulated limits for use in food contact materials and products. For example, Dibutyl phthalate (DBP) is strictly prohibited as a food additive, yet it may be used in PVC and rubber-based materials at a maximum content of 5%, with a specific migration limit (SML) of 0.3 mg/kg.
In March 2025, China issued the National Food Safety Standard GB 31604.30–2025 – Determination of Phthalate Compounds and Their Migration in Food Contact Materials and Articles, which added the determination of Diisodecyl phthalate (DIDP). The new standard specifies gas chromatography–mass spectrometry (GC–MS) as the analytical technique for 19 phthalate esters, namely: DMP, DEP, DAP, DIBP, DBP, DMEP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DEHP, DPhP, DNOP, DINP, DIDP, and DNP (see Table 1).
In this study, the EXPEC 3750 GC–MS was used to analyze the content and migration levels of these 19 phthalates in food contact materials. The method achieved excellent performance, with correlation coefficients (R²) ranging from 0.9921 to 0.9998, high-concentration precision between 0.84% and 2.23%, and low-concentration precision between 0.52% and 4.14%. The limits of detection (LOD) were 4.40–317.60 μg/kg for phthalates in food contact materials, and 0.88–63.52 μg/L for their migration levels.
Keywords: EXPEC 3750 GC–MS, phthalate analysis, PAEs detection, food contact materials, phthalate migration, GC–MS food safety testing, plasticizer determination, packaging contaminant analysis, gas chromatography mass spectrometry, phthalate ester quantification, food safety instrument, GC–MS application.
Instruments and Reagents
Instruments:Gas Chromatography–Mass Spectrometer (EXPEC 3750 GC-MS, Hangzhou EXPEC Technology Co., Ltd.)

EXPEC 3750 GC-MS Appearance
Reagents and Materials
Standard stock solutions of 19 phthalate esters (PAEs) at 100 μg/mL concentration (see Table 1). n-Hexane (chromatographic grade). High-purity helium gas (purity ≥ 99.999%).
Table 1. Basic Information of 19 Phthalate Esters (PAEs)
| NO. | Target Compounds | Abbreviation | CAS NO. | Quantitative Ion (m/z) | Qualitative Ion (m/z) |
| 1 | Dimethyl phthalate | DMP | 131-11-3 | 163 | 77 |
| 2 | Diethyl phthalate | DEP | 84-66-2 | 149 | 177 |
| 3 | Diallyl phthalate | DAP | 131-17-9 | 149 | 41 |
| 4 | Diisobutyl phthalate | DIBP | 84-69-5 | 149 | 223 |
| 5 | Dibutyl phthalate | DBP | 84-74-2 | 149 | 223 |
| 6 | Di(2-methoxyethyl) phthalate | DMEP | 117-82-8 | 149 | 59 |
| 7 | Di(4-methyl-2-pentyl) phthalate | BMPP | 146-50-9 | 149 | 167 |
| 8 | Di(2-ethoxyethyl) phthalate | DEEP | 605-54-9 | 149 | 72 |
| 9 | Dipentyl phthalate | DPP | 131-18-0 | 149 | 237 |
| 10 | Dihexyl phthalate | DHXP | 84-75-3 | 149 | 251 |
| 11 | Butyl benzyl phthalate | BBP | 85-68-7 | 149 | 91 |
| 12 | Di(2-butoxyethyl) phthalate | DBEP | 117-83-9 | 149 | 101 |
| 13 | Dicyclohexyl phthalate | DCHP | 84-61-7 | 149 | 167 |
| 14 | Di(2-ethylhexyl) phthalate | DEHP | 117-81-7 | 149 | 167 |
| 15 | Diphenyl phthalate | DPhP | 84-62-8 | 225 | 77 |
| 16 | Di-n-octyl phthalate | DNOP | 117-84-0 | 149 | 127 |
| 17 | Diisononyl phthalate | DINP-1 | 28553-12-0 | 149 | 293 |
| Di-C₈–C₁₀ branched alkyl phthalate (mainly C₉)) | DINP-2 | 68515-48-0 | 149 | 293 | |
| 18 | Diisodecyl phthalate | DIDP-1 | 26761-40-0 | 149 | 307 |
| Di-C₉–C₁₁ branched alkyl phthalate (mainly C₁₀) | DIDP-2 | 68515-49-1 | 149 | 307 | |
| 19 | Dinonyl phthalate | DNP | 84-76-4 | 149 | 293 |
Note: When determining diisononyl phthalate (DINP) and di-C₈–C₁₀ branched alkyl phthalate (mainly C₉), as well as diisodecyl phthalate (DIDP) and di-C₉–C₁₁ branched alkyl phthalate (mainly C₁₀) in plastic sample test solutions, the standard materials DINP-1 and DIDP-1 should be used. For rubber, adhesives, coatings, paints, paper, and paperboard or other sample matrices, the standard materials DINP-2 and DIDP-2 should be selected.
Experimental Method
Standard Solutions:Using n-hexane as the solvent, nineteen phthalate ester (PAEs) standard stock solutions were serially diluted to prepare calibration standards at concentrations of 0.05 μg/mL, 0.1 μg/mL, 0.2 μg/mL, 0.5 μg/mL, 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, and 20 μg/mL.
For curve fitting:
- DINP and DIDP were calibrated using 1, 2, 5, 10, and 20 μg/mL concentrations.
- DNOP and DNP were calibrated using 0.1, 0.2, 0.5, 1, and 2 μg/mL concentrations.
- The remaining 15 compounds were calibrated using 0.05, 0.1, 0.2, 0.5, and 1 μg/mL concentrations.
Instrument Parameters
The key operating parameters of the EXPEC 3750 GC–MS system are shown in Table 2.
Table 2. Reference Conditions for Gas Chromatography–Mass Spectrometry (GC-MS)
| Module | Component | Parameter |
| GC | Inlet Temperature | 280 ℃ |
| Injection Volume | 1 μL | |
| Injection Mode | Splitless | |
| Column | DB-5MS, 30 m × 0.25 mm × 0.25 µm | |
| Carrier Gas Flow Rate | 1 mL/min,constant flow | |
| Oven Temperature Program | Initial 60 ℃,hold for 2 min; increase at 20 °C/min to 220 °C, hold for 2 min; then ramp at 5 °C/min to 250 °C, hold for 2 min; increase at 20 °C/min to 290 °C, hold for 6 min. | |
| MS | GC–MS Interface Temperature | 280 ℃ |
| Ion Source Temperature | 280 ℃ | |
| Scan Mode | EI, SIM (See table 1) |
Results and Discussion
Figure 2 shows the chromatographic separation of the 19 phthalate esters (PAEs) on the analytical column. Figures 3 and 4 display the characteristic ion chromatograms of DINP and DIDP, respectively.
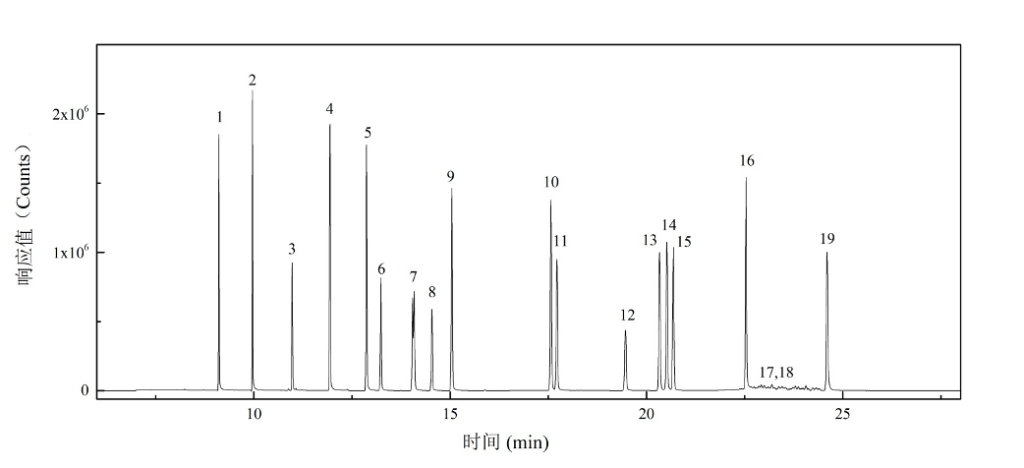
Figure 2. The total ion chromatogram (TIC) of the 19 PAEs.
Note:1-DMP;2-DEP;3-DAP;4-DIBP;5-DBP;6-DMEP;7-BMPP;8-DEEP;9-DPP;10-DHXP;11-BBP;12-DBEP;13-DCHP;14-DEHP;15-DPhP;16-DNOP;17-DINP;18-DIDP;19-DNP
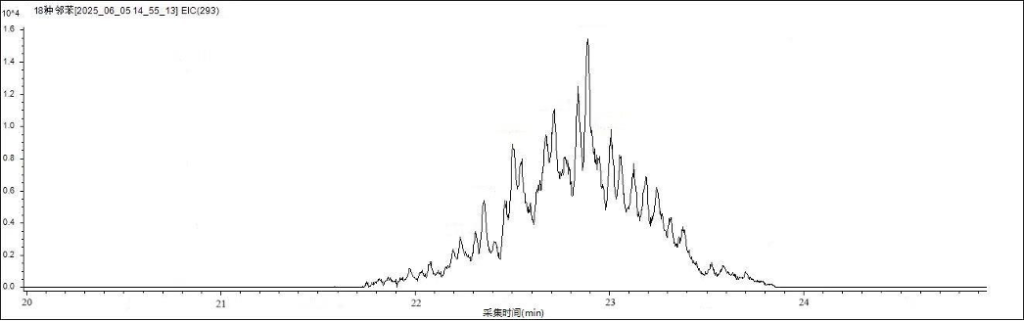
Figure 3. Characteristic ion chromatogram of DINP.
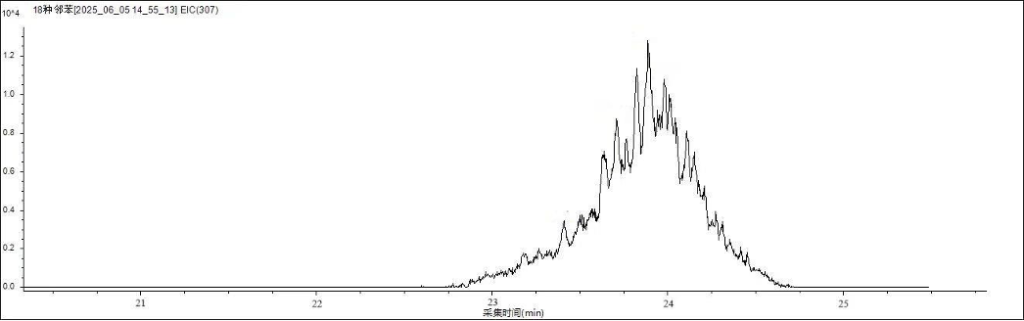
Figure 4. Characteristic ion chromatogram of DIDP
Calibration Curve
According to the standard method, quantification was performed using the external standard method. For DINP and DIDP in the range of 1 μg/mL–20 μg/mL, the correlation coefficients (R²) were 0.9991 and 0.9992, respectively.For DNOP and DNP in the range of 0.1 μg/mL–2 μg/mL, the R² values were 0.9935 and 0.9922, respectively.For the remaining 15 PAEs in the range of 0.05 μg/mL–1 μg/mL, the R² values were all greater than 0.9921.Therefore, the linear correlation coefficients (R²) of all analytes were between 0.9921 and 0.9998. The calibration curves of selected compounds are shown in Figure 5, and detailed data are provided in Table 3.
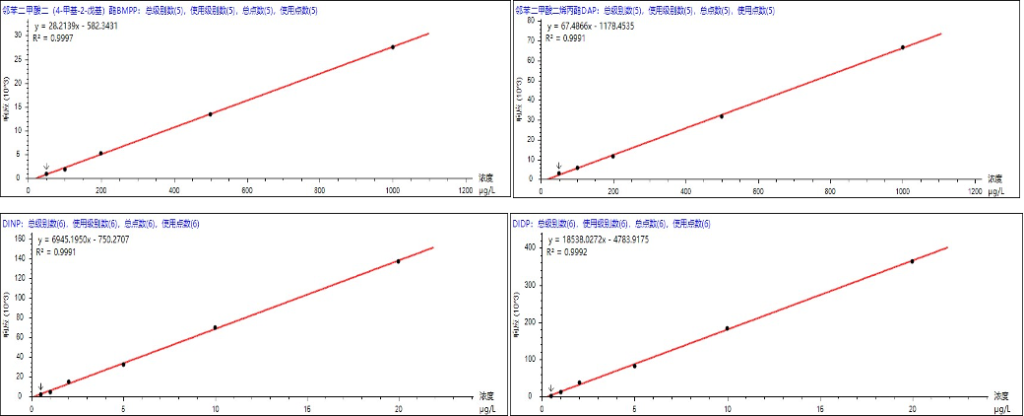
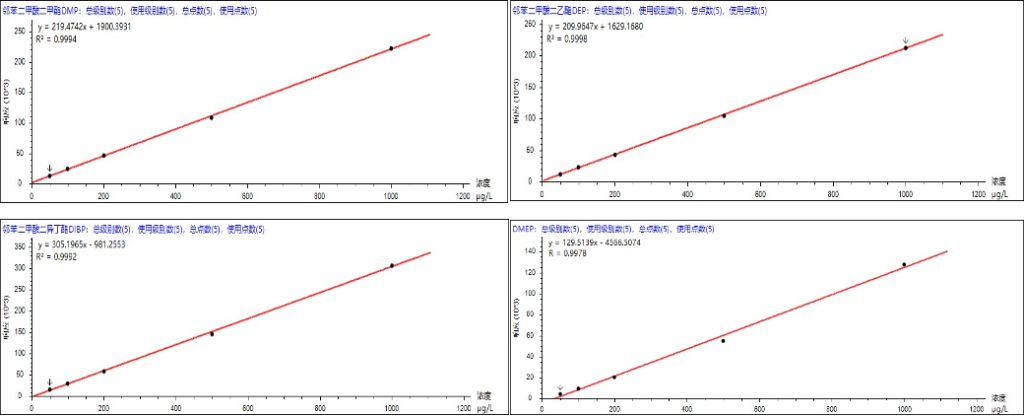
Figure 5. Standard calibration curves of selected PAEs.
Method Precision
According to the experimental analytical method, seven consecutive analyses were performed on samples with concentrations of 1 μg/mL and 0.05 μg/mL, representing high and low concentration levels, respectively, to evaluate method precision. The results are shown in Table 3. The precision (RSD) for the 19 PAEs at high concentration ranged from 0.84% to 2.23%, while at low concentration it ranged from 0.52% to 4.14%, indicating good stability and repeatability of the instrumental method.
Table 3. Linear correlation coefficients and method precision of 19 PAEs.
| Target Compounds | Remaining time(min) | R2 | Low-concentration method precision (%) | High-concentration method precision (%) | |
| Dimethyl phthalate | DMP | 9.11 | 0.9994 | 1.89 | 1.68 |
| Diethyl phthalate | DEP | 9.96 | 0.9998 | 1.47 | 2.07 |
| Diallyl phthalate | DAP | 10.97 | 0.9991 | 1.64 | 1.73 |
| Diisobutyl phthalate | DIBP | 11.93 | 0.9992 | 1.27 | 2.08 |
| Dibutyl phthalate | DBP | 12.87 | 0.9984 | 1.14 | 2.16 |
| Di(2-methoxyethyl) phthalate | DMEP | 13.23 | 0.9978 | 2.53 | 1.25 |
| Di(4-methyl-2-pentyl) phthalate | BMPP | 14.08 | 0.9997 | 3.69 | 1.88 |
| Di(2-ethoxyethyl) phthalate | DEEP | 14.53 | 0.9946 | 4.14 | 1.37 |
| Dipentyl phthalate | DPP | 15.04 | 0.9981 | 1.30 | 0.84 |
| Dihexyl phthalate | DHXP | 17.56 | 0.9951 | 0.54 | 2.07 |
| Butyl benzyl phthalate | BBP | 17.71 | 0.9925 | 1.22 | 1.92 |
| Di(2-butoxyethyl) phthalate | DBEP | 19.46 | 0.9921 | 3.49 | 1.72 |
| Dicyclohexyl phthalate | DCHP | 20.33 | 0.9932 | 0.90 | 1.97 |
| Di(2-ethylhexyl) phthalate | DEHP | 20.51 | 0.9964 | 0.52 | 1.84 |
| Diphenyl phthalate | DPhP | 20.68 | 0.9978 | 1.51 | 1.47 |
| Di-n-octyl phthalate | DNOP | 22.53 | 0.9935 | 1.24 | 2.16 |
| Diisononyl phthalate | DINP | 21.86~ 23.12 | 0.9991 | 2.25 | 2.07 |
| Diisodecyl phthalate | DIDP | 23.12~ 24.57 | 0.9992 | 2.43 | 2.23 |
| Dinonyl phthalate | DNP | 24.60 | 0.9922 | 1.26 | 1.56 |
Method Detection Limits (MDL)
Detection Limits of Phthalates in Food Contact Materials
According to the standard requirements, when the sample weight was 0.2 g and the final volume was 1 mL, test solutions were prepared for detection limit analysis as follows:
- 15 PAEs at 0.25 mg/kg,
- DINP and DIDP at 5 mg/kg,
- DNP and DNOP at 0.5 mg/kg.
Each sample was analyzed seven times in parallel under the reference instrumental conditions, and the standard deviation (S) was calculated. The method detection limit (MDL) was then determined using the formula: MDL = 3.143 × S (confidence level 99%). As shown in Table 4, the MDLs for the 19 PAEs in food contact materials ranged from 4.40 μg/kg to 317.60 μg/kg.
Detection Limits of Phthalate Migration from Food Contact Materials
According to the standard procedure, this study examined the method detection limits for aqueous, acidic, and ethanol-based (ethanol volume fraction < 50%) food simulants.Test solutions were prepared as follows:
- 15 PAEs at 0.05 μg/mL,
- DINP and DIDP at 1 μg/mL,
- DNP and DNOP at 0.1 μg/mL.
Each solution was analyzed seven times in parallel under the reference GC–MS conditions, and the standard deviation (S) was calculated. Using the formula MDL = 3.143 × S (confidence level 99%), the method detection limits were obtained. As shown in Table 4, the MDLs for the 19 PAEs in migration testing ranged from 0.88 μg/L to 63.52 μg/L.
Table 4. Method Detection Limits (MDL) of 19 Phthalate Esters (PAEs)
| No. | Target Compound | Abbreviation | Retention Time (min) | Food Contact Materials (μg/kg) | Migration (μg/L) |
| 1 | Dimethyl phthalate | DMP | 9.11 | 13.65 | 2.73 |
| 2 | Diethyl phthalate | DEP | 9.96 | 10.10 | 2.02 |
| 3 | Diallyl phthalate | DAP | 10.97 | 12.85 | 2.57 |
| 4 | Diisobutyl phthalate | DIBP | 11.93 | 10.10 | 2.02 |
| 5 | Dibutyl phthalate | DBP | 12.87 | 9.10 | 1.82 |
| 6 | Di(2-methoxyethyl) phthalate | DMEP | 13.23 | 19.15 | 3.83 |
| 7 | Di(4-methyl-2-pentyl) phthalate | BMPP | 14.08 | 32.05 | 6.41 |
| 8 | Di(2-ethoxyethyl) phthalate | DEEP | 14.53 | 21.95 | 4.39 |
| 9 | Dipentyl phthalate | DPP | 15.04 | 35.05 | 7.01 |
| 10 | Dihexyl phthalate | DHXP | 17.56 | 4.40 | 0.88 |
| 11 | Butyl benzyl phthalate | BBP | 17.71 | 7.80 | 1.56 |
| 12 | Di(2-butoxyethyl) phthalate | DBEP | 19.46 | 23.55 | 4.71 |
| 13 | Dicyclohexyl phthalate | DCHP | 20.33 | 7.15 | 1.43 |
| 14 | Di(2-ethylhexyl) phthalate | DEHP | 20.51 | 4.50 | 0.90 |
| 15 | Diphenyl phthalate | DPhP | 20.68 | 11.75 | 2.35 |
| 16 | Di-n-octyl phthalate | DNOP | 22.53 | 44.75 | 8.95 |
| 17 | Diisononyl phthalate | DINP | 21.86–23.12 | 306.55 | 61.31 |
| 18 | Diisodecyl phthalate | DIDP | 23.12–24.57 | 317.60 | 63.52 |
| 19 | Dinonyl phthalate | DNP | 24.60 | 51.70 | 10.34 |
Actual Sample Analysis
Phthalate Content in Food Contact Materials
According to the standard procedure, beverage plastic bottles A and B were cut into small pieces with scissors. Each sample (0.2 g) was weighed and placed in a flask with 10 mL of tetrahydrofuran (THF), followed by ultrasonic extraction for 30 minutes. After ultrasonication, 40 mL of n-hexane was added, the mixture was vortexed, and centrifuged to collect the supernatant. The supernatant was evaporated to dryness under a nitrogen stream and reconstituted to 1 mL with n-hexane. The solution was filtered through a 0.45 μm organic membrane filter and analyzed.
Phthalate Migration from Food Contact Materials
Following the standard method, 50% (v/v) ethanol was prepared as the food simulant. The beverage plastic bottles A and B were filled with the simulant, sealed, and kept stationary for 7 days. After migration, 10 mL of the soaking solution was accurately transferred to a 25 mL centrifuge tube, followed by the addition of 4 mL of n-hexane. The mixture was vortexed for 10 minutes, centrifuged, and the n-hexane layer was collected. This extraction was repeated twice, and the extracts were combined. The combined extract was evaporated to dryness under nitrogen and redissolved in 1 mL of n-hexane, filtered through a 0.45 μm organic membrane, and analyzed.
Results:The analysis of phthalates (PAEs) in food contact materials detected DIBP, DBP, and DEEP, with concentrations ranging from 21.39 μg/kg to 104.21 μg/kg. No PAEs were detected in the migration tests of either beverage bottle sample. Detailed results are shown in the table below.
Table 5. Phthalate Esters and Their Migration in Food Contact Materials (Unit: μg/kg)
| Target Compounds | Sample A | Sample B | Migration – Sample A | Migration – Sample B | |
| Diisobutyl phthalate | DIBP | 21.39 | 35.99 | ND | ND |
| Dibutyl phthalate | DBP | 50.46 | 104.21 | ND | ND |
| Di(2-ethoxyethyl) phthalate | DEEP | 74.15 | 83.73 | ND | ND |
ND: Not detected.
Conclusion
Using the EXPEC 3750 GC–MS, nineteen phthalate esters (PAEs) and their migration levels in food contact materials and articles were analyzed. The correlation coefficients (R²) for the 19 compounds ranged from 0.9921 to 0.9998. The precision at high concentration ranged from 0.84% to 2.23%, and at low concentration from 0.52% to 4.14%. The method detection limits were 4.40–317.60 μg/kg for PAEs in food contact materials and 0.88–63.52 μg/L for PAE migration. The method demonstrated high precision, good stability, and strong reliability, providing solid technical support for the determination of both phthalate content and migration levels in food contact materials
Two beverage bottle samples were selected for real sample analysis. Results showed that DIBP, DBP, and DEEP were detected in the contact materials, with concentrations ranging from 21.39 μg/kg to 104.21 μg/kg. No PAEs were detected in the migration tests.
(1) The linear correlation coefficients (R²) of all 19 PAEs were greater than 0.9921.
(2) The precision at high concentrations ranged from 0.84% to 2.23%, and at low concentrations from 0.52% to 4.14%.
(3) The detection limits of the 19 PAEs in food contact materials ranged from 4.40 to 317.60 μg/kg, while those for migration tests ranged from 0.88 to 63.52 μg/L.

