Injections refer to sterile solutions (including emulsions and suspensions) made of drugs for injection into the body, as well as sterile powders or concentrated solutions that are prepared into solutions or suspensions before use. Injections act quickly and reliably, and are not affected by pH, enzymes, food, etc., and can play a systemic or local positioning role. They are suitable for patients who are not suitable for oral medication and who cannot take oral medications. However, the development and production process of injections is complex, and safety and body adaptability have become potential issues. risk factors. One of the very important safety risk factors is the content of impurity elements. When the elemental impurities in injections exceed the maximum allowable daily exposure (PDE), they will cause harm to the human body.
The Q3D elemental impurity guidelines issued by the International Conference on Harmonization of Technical Registration of Pharmaceuticals for Human Use (ICH) stipulate PDEs for 24 elements. As a member state of ICH, China is also vigorously promoting the implementation of ICH Q3D . ICH Q3D divides elements into three categories based on their toxicity and likelihood of appearing in pharmaceuticals. Among them, Class 1 elements include As, Cd, Hg, and Pb. These elements are highly toxic and are usually derived from mineral excipients. They must not be used or are restricted in use in pharmaceutical production. Therefore, Class 1 elements must be evaluated in risk assessments for all routes of administration . Category 2A elements include Co, Ni, and V. These elements are more likely to appear in preparations and require risk assessment of all potential sources and routes of administration.
ICH Q3D recommends the use of inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) for impurity element analysis. However, for the determination of elements such as Cd, As, and Hg, which are very low in content and must be detected, ICP-OES is not competent due to its high detection limit, so ICP-MS has become the preferred method . This article is based on the SUPEC 7000 ICP-MS and uses the undiluted direct injection method to establish physiological saline (0.9% sodium chloride) and lactated Naringer injection (containing sodium lactate, sodium chloride, potassium chloride and calcium chloride). This method is a method for the determination of Class 1 and 2A elements. This method has good linearity, accuracy and precision, and can maintain good matrix tolerance in the direct injection of the above-mentioned high-matrix samples. It can fully meet the requirements for impurities in injection samples. Analysis requirements for elements.
Keywords: medicine, ICH Q3D, injection, impurity elements, ICP-MS, physiological saline
Experimental part
instrument
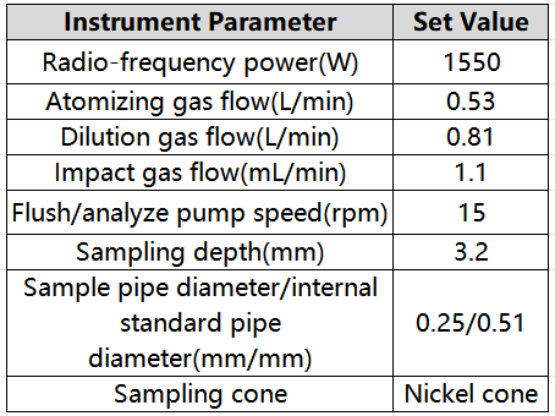
Table 1 Inductively coupled plasma mass spectrometer

Table 2 Detection parameters of inductively coupled plasma mass spectrometer
Reagents and standards
Reagents: nitric acid (excellent grade pure).
Pure water: 18.2 MΩ·cm deionized water.
Standard solution: V, Co, Ni, As, Cd, Hg, Pb, Sc, Ge, In, Re, Au single element standard solution (1000 ug/mL, National Institute of Nonferrous Metals).
Sample pretreatment: Physiological saline and sodium lactate Ringer's injection do not need to be diluted and can be injected directly for analysis.
Test Results
Standard curve and detection limit: By adding internal standard online, a standard curve is established in helium collision mode. According to the sample analysis steps, the sample concentration corresponding to 3 times the standard deviation of the measured value obtained from 11 consecutive analyzes of the blank sample is used as the method detection limit.
Accuracy and precision: Normal saline and sodium lactate Ringer's injection were measured, and three spike concentrations of low, medium, and high were used for each element to conduct a spike recovery test. The accuracy of the method was measured by the spike recovery rate. The measurement was repeated seven times independently using standard samples, and the RSD of the measurement results was calculated to examine the precision of the method. The spike recovery rate is between 88.6 and 105.7%, and the RSD is less than 5.6%.

Matrix tolerance test
In order to examine the tolerance of injection samples with high matrix during long-term injection, normal saline (0.9% sodium chloride matrix) spiked samples were continuously injected for 3 hours and measured every 20 minutes. The instrument was judged based on the trend of the measured values. stability. The results are shown in Table 4 and Figure 1. During the 3 hours of continuous injection, the trend of the measured values of each element was stable, and the RSD was less than 4.7%.
Table 4 Continuous injection and measurement results of physiological saline (μg/L)
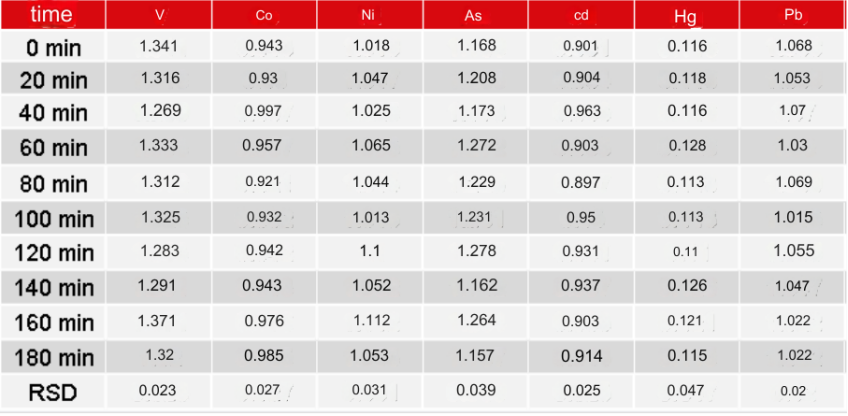
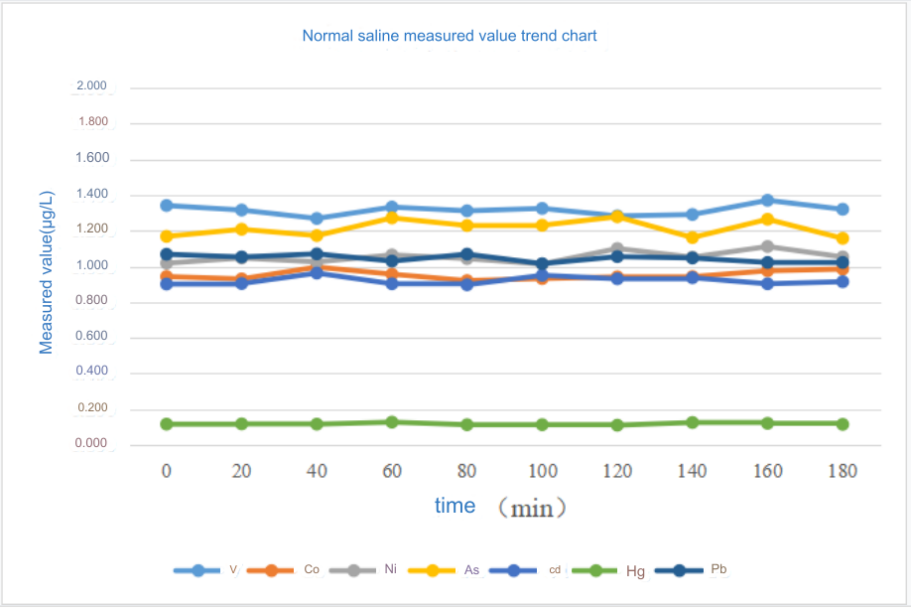
Figure 1 Trend chart of measured values of continuous injection of physiological saline
Conclusion
This article established an analysis method for Class 1 and Class 2A impurity elements in physiological saline and sodium lactate Ringer's injection based on the SUPEC 7000 ICP-MS. This method has good linearity, accuracy, precision and matrix tolerance, and can fully meet the analysis needs of impurity elements in high-matrix injection samples.
appendix
Equipment and consumables solutions
1. Configuration details of SUPEC 7000 high-salt resistant sampling system
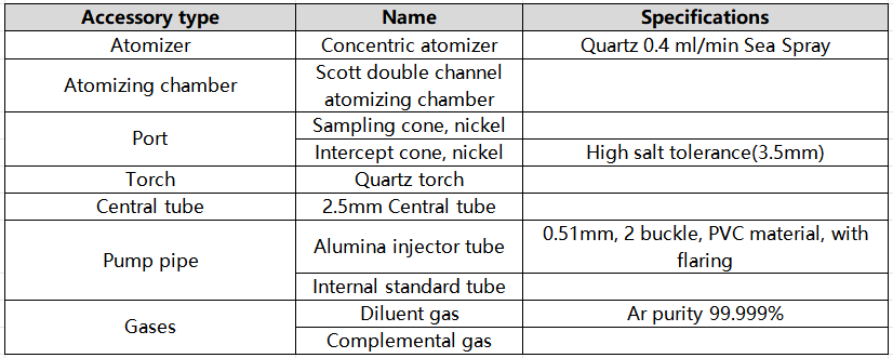
2. Reagents and standards


