Mercury is a toxic element that can cause severe damage to human nerves and kidneys. It occurs in nature in the form of inorganic mercury (Hg2+), methylmercury (MeHg) and ethylmercury (EtHg). The toxicity of mercury mainly depends on its concentration and chemical form. Organic mercury is more toxic than inorganic mercury. Among organic mercury, methylmercury is the most toxic and can cause irreversible damage to the human central nervous system.
Heavy metal pollution in water is one of the most important environmental problems. Mercury content in water is extremely low but can be enriched and amplified through the food chain, causing harm to human health. The limit on the total amount of mercury cannot scientifically and accurately reflect the toxic effects of mercury on the human body, and different forms of mercury need to be detected. Therefore, the analysis of mercury speciation in water bodies is of great significance.
In this experiment, SUPEC 7000 ICP-MS coupled with liquid chromatography was used to analyze the mercury forms in tap water, and the detection limit, precision and spiked recovery of actual samples were obtained.
Experimental part
instrument
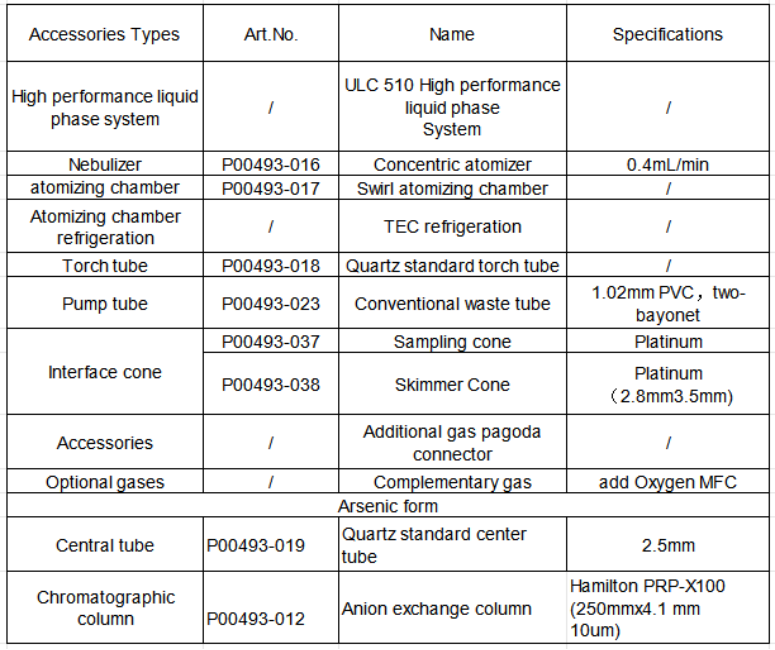
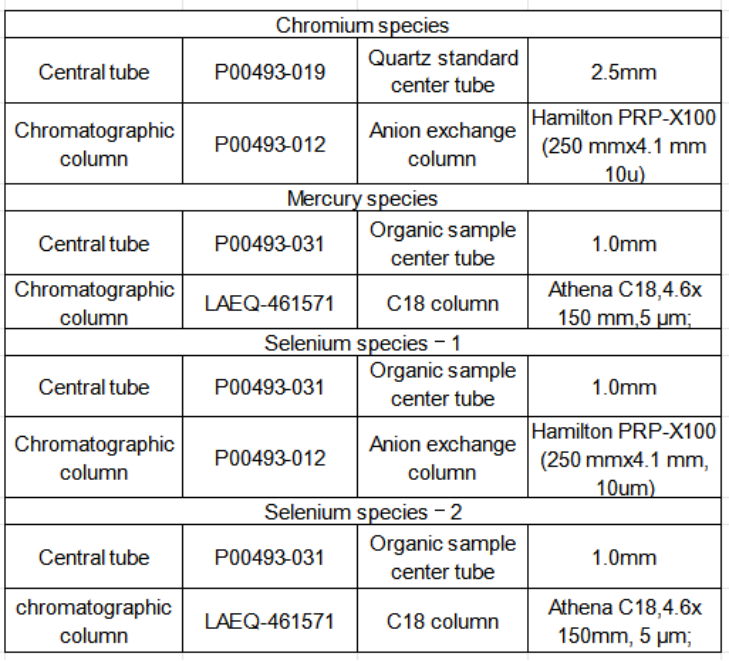
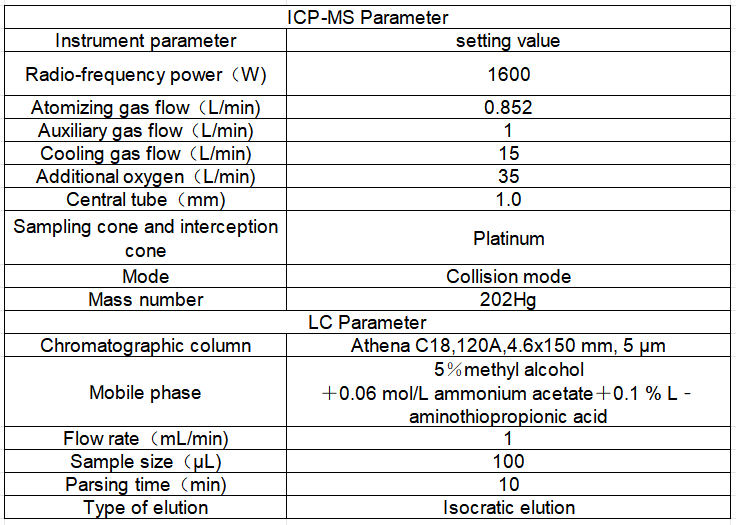
Table 1 Liquid chromatography-inductively coupled plasma mass spectrometry
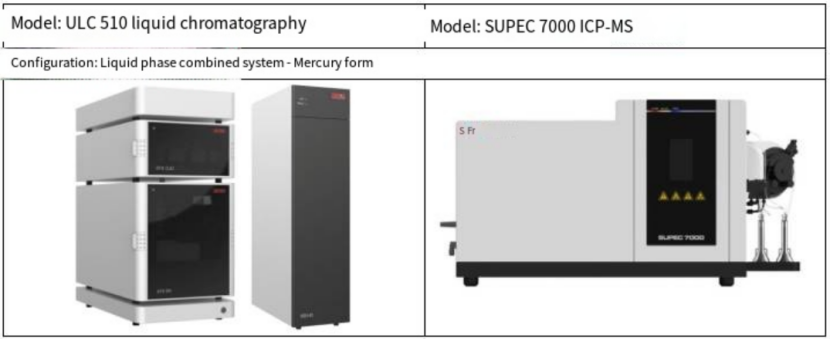
Table 2 Inductively coupled plasma mass spectrometer detection parameters
Reagents and standard solutions
Primary water: 18.2 MΩ·cm deionized water
Inorganic mercury standard solution (concentration 1000 mg•L-1, measured in substance, National Nonferrous Metals and Electronic Materials Analysis and Testing Center)
Methylmercury chloride standard solution (concentration 1000 mg•L-1, based on substance, CNW)
Ethyl mercuric chloride standard solution (concentration 1000 mg•L-1, based on substance, CNW)
Ammonium acetate (≥99.0%, Aladdin)
L-cysteine (≥98.0%, CNW)
Sodium hydroxide (99.99%, Aladdin)
Methanol (chromatographic grade, CNW)
Sample preparation
Tap water is filtered through a 0.45 μm organic filter membrane and directly measured on the machine.
Results and discussion
Chromatographic separation
FIG1 shows the chromatographic separation spectrum at the 5 μg/L mark. It can be seen from the figure that the three types of mercury are well separated by baseline. The retention time of each form is shown in Table 4.
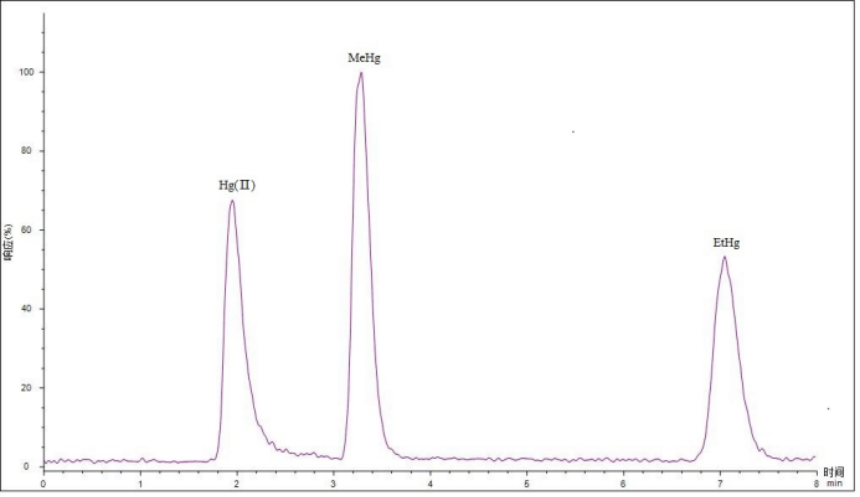
Fig.1 Chromatographic separation spectrum of 5μg/L mark point
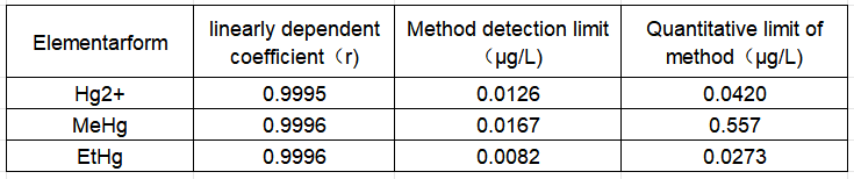
Table 3 Linear correlation coefficients, method detection limits and quantification limits of three mercury forms
Precision test
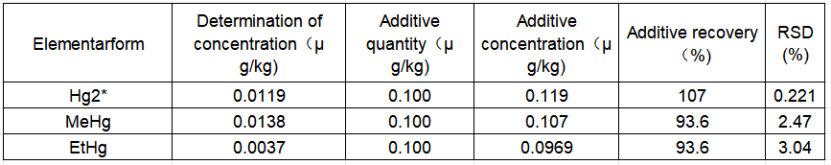
Since mercury was not detected in the sample, a certain concentration of mixed standard solution of mercury forms was added to the water sample, and three sample solutions were measured and RSD was calculated. The sample data and precision are shown in Table 6. It can be seen from the table that RSD is less than 2.95%, and the method has good precision.
Table 4 Sample precision test results (μg/kg)
Spike recovery test
In order to investigate the reliability of the ultrasonic waterbath extraction method, a certain amount of mercury form standard solution was added to the water sample, mixed evenly and placed overnight, and then extracted by ultrasonic. The spike recovery test results are shown in Table 6. As can be seen from the table, the spike recovery of the water sample was between 92.0% and 107%.
Table 5 Sample spike recovery test results
in conclusion
This experiment uses SUPEC7000 ICP-MS coupled with liquid chromatography to achieve quantitative analysis of three forms of mercury in tap water. The detection limits of inorganic mercury, methylmercury, and ethylmercury are 0.0126 μg•L-1, 0.0167 μg•L-1, and 0.0082 μg•L-1, respectively. The spiked recovery of the samples is between 92.0% and 107.4%, and the precision is less than 3.04%. This method has a low detection limit, high accuracy, and good precision, and can meet the requirements of LC-ICP-MS for the analysis of three forms of mercury in tap water.
Equipment and consumables solutions