Rare earth is known as "industrial monosodium glutamate", "industrial vitamin" and "mother of new materials". It is a precious strategic metal resource with irreplaceable excellent magnetic, optical and electrical properties. It plays a huge role in improving material properties and increasing product varieties. Due to their great effect and low dosage, rare earths have become an important element to improve product structure, increase technological content, and promote technological progress in the industry. They are widely used in metallurgy, military, petrochemical, glass ceramics, agriculture, and new materials. Therefore, the demand for high-purity REE materials is becoming more and more stringent. When other REE elements exist, it will often affect the function of the final product. Therefore, the detection method of high-purity rare earths is very important.
The application center uses the triple quadrupole (ICP-MS/MS) to realize the determination of elements with less interference in collision mode, the determination of Gd elements in oxygen mass transfer mode and the determination of Tb elements in ammonia mass transfer mode. The method is simple in operation, stable in test and high in efficiency, and provides ideas and reference for laboratories to accurately test and analyze REE impurity elements in high-purity REE.
Keywords: ICP-MS/MS, metallurgy, materials, cerium carbonate, collision reaction cell, high-matrix high-salt configuration, ammonia mass-shift mode, oxygen mass-shift mode
Experimental part
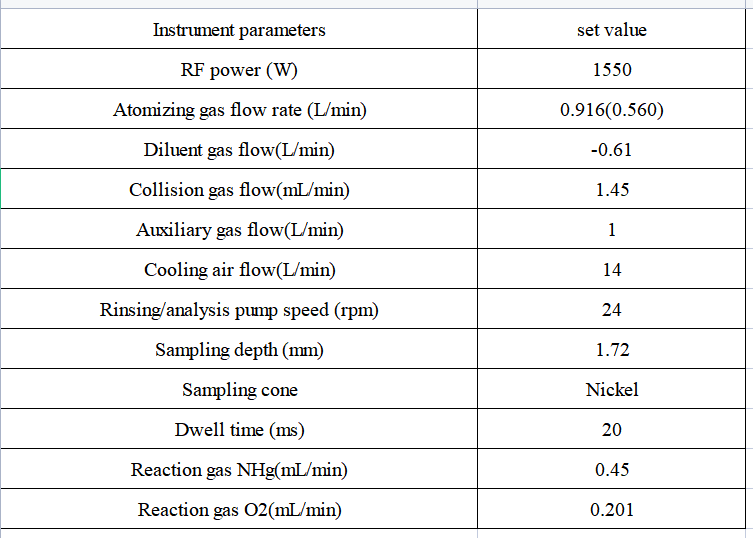
Instrument: EXPEC 7350 ICP-MS/MS, equipped with standard quartz sampling system
Table 1 Inductively coupled plasma mass spectrometer instrument

Table 2 Detection parameters of inductively coupled plasma mass spectrometer
Reagents and Standards
Reagent: superior pure nitric acid; Remarks: higher purity reagents (G3 grade) can be purchased
Pure water: 18.25 MΩ·cm deionized water;
Standard solution: La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y multi-element standard solution, 100 μg/mL, National Institute of Nonferrous Metals.
Sample Handling
Accurately weigh 0.1 g of cerium carbonate powder sample (accurate to 0.001 g) into a polytetrafluoroethylene digestion cup, slowly add 4 mL of nitric acid, shake gently to mix, and heat at 160 °C for about 1h on the electric heating plate. It was completely digested, and after cooling, it was transferred to a 100 mL PP bottle with ultrapure water to dilute to a constant volume, and then tested on the machine.
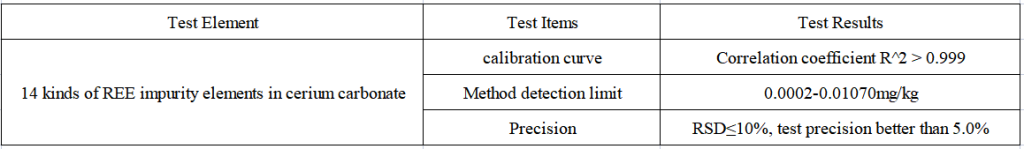
Test Results
Epilogue
In this paper, 14 kinds of REE impurity elements in high-purity cerium carbonate sample solution were successfully measured by ICP-MS/MS. For elements with less Ce matrix interference, the superior collision cell can be used to eliminate the interference. For elements with greater Ce matrix interference, such as Tb using ammonia mass transfer mode and Gd using oxygen mass transfer mode to achieve the stability of the element test analysis. They use the extra quadrupole of ICP-MS/MS, which can select the target mass to enter the reaction cell, precisely control and monitor the reaction process in the complex matrix, and effectively eliminate the polyatomic interference in the Ce matrix, so as to realize good sample test analysis for cerium carbonate.
Appendix
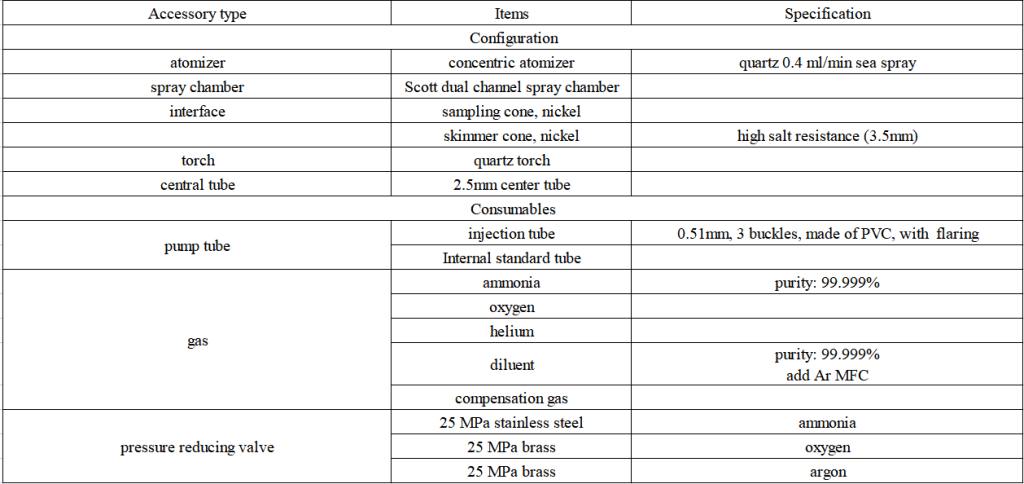
Equipment and Consumables Solutions
1.EXPEC 7350 standard sampling system configuration details
2.Reagents and Standards


