N-nitrosamine dimethylamine (NDMA) is a compound with an N-N=O structure that reacts with DNA to alkylate and cause cancer. The FDA has revealed that the finished gastric drug ranitidine contains nitrosamine carcinogen N-nitrosamine dimethylamine that exceeds the limit requirements, and the FDA issued an announcement to recall ranitidine products on the US market, which mentioned that the NDMA content in some products will increase over time, which may lead to higher than acceptable levels of impurities.
Many domestic and foreign pharmaceutical manufacturers have initiated recalls due to the detection of over-limit NDMA in the digestive system drug ranitidine. Therefore, regulators and manufacturers need an analytical testing method to detect N-nitrosamine dimethylamine in such drugs to effectively protect the safety of people's medications.
The LC-MS/MS method has the advantages of high sensitivity, high resolution, rapid analysis, and accurate results, which can reduce the possibility of false negatives or false positives caused by matrix interference, and can meet the strict sensitivity, specificity and accuracy requirements of drug detection. In this paper, the SP.E. EXPEC 5210 triple quadrupole LC/MS system was developed to develop an LC-MS/MS method for APCI sources for the testing and quantification of N-nitrosamine dimethylamine in ranitidine raw capsules and tablets.
Experimental part
- Standard N-nitrosamine dimethylamine standard purchased from China Food and Drug Control Institute and stored in a -20°C refrigerator.
- Sample ranitidine hydrochloride capsules, labeled 136#, 105#, 104#, 61#, 12#
- Reagents methanol is chromatographic grade, purchased from Merck, formic acid is LC-MS grade, purchased from Ampera.
- Instruments ULC 510 ultra-high performance liquid chromatograph (specifically equipped with binary ultra-high pressure infusion pump, ultra-high pressure autosampler, column oven), EXPEC 5210 triple quadrupole tandem mass spectrometer.

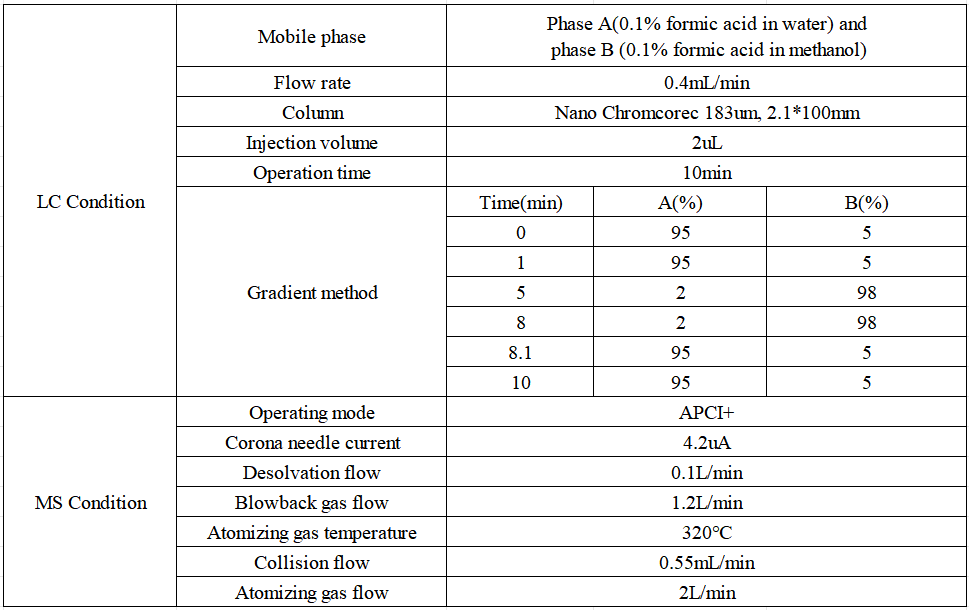
Liquid phase and mass spectrometry conditions
The monitoring mode is multi-reaction monitoring (MRM), and the N-nitrosodimethylamine compound monitors the ion pair, cone voltage, collision energy and other parameters as shown in the figure below.
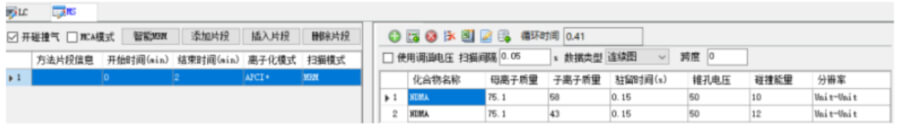
Sample preparation
Refer to mass spectrometry (Chinese Pharmacopoeia 2015 Edition IV General Principles 0431) determination, the specific pretreatment steps are as follows:
Preparation of ranitidine hydrochloride capsule test solution: take an appropriate amount of capsule content, equivalent to ranitidine 300mg, into a 50mL centrifuge tube, add methanol 10mL precisely, vortex and mix well for 1min, then shake for 40min, centrifuge, take the supernatant and filter, and take the filtrate to obtain.
Experimental results
- Linear Accurately weigh an appropriate amount of N-nitrosodimethylamine reference substance, add methanol to dissolve and quantitatively dilute to make 1mL of solution containing 1, 2, 5, 5, 10, 100, 250, 500ng. According to the above method, the standard curve after being fitted by the external standard method is shown in Figure 1 below, and the spectral overlay of different concentrations of N-nitrosodimethylamine is shown in Figure 2.
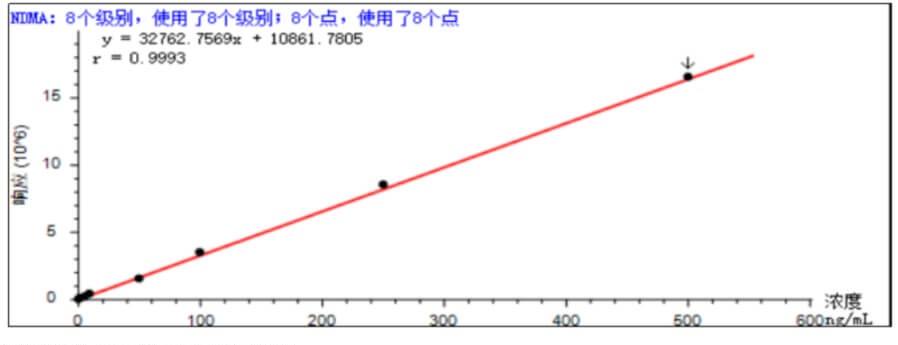
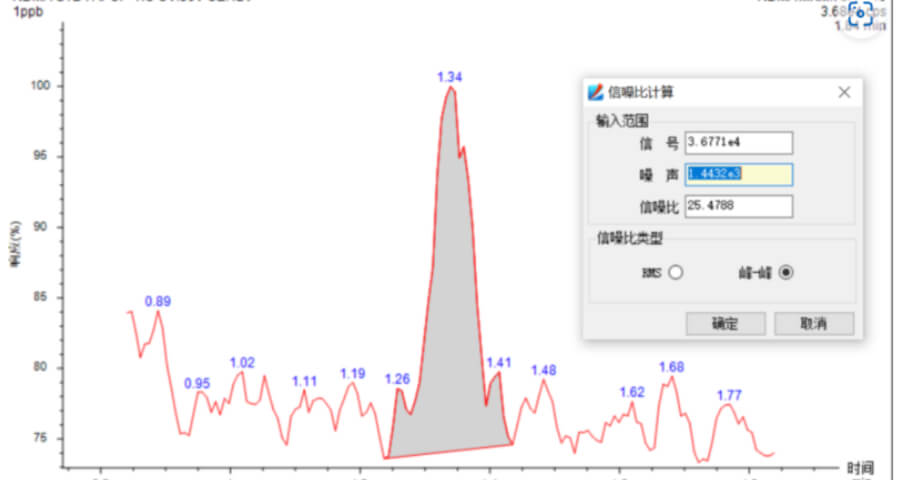
- Sensitivity Accurately weigh an appropriate amount of N-nitrosodimethylamine reference substance, add methanol to dissolve and quantitatively dilute to make a solution containing 1ng in 1mL, and the signal-to-noise ratio of the main peak of the chromatography test was not less than 10.
- Stability Accurately weigh an appropriate amount of N-nitrosodimethylamine reference substance, add methanol to dissolve and quantitatively dilute to make a solution containing 2ng in 1mL, repeat the injection 6 times, and the RSD value of the peak area is 2.34%, which meets the requirements that the relative standard deviation of the obtained peak area should not exceed 10%.
- Spiked recovery The content of N-nitrosodimethylamine in ranitidine hydrochloride capsule sample 136# was not detected as a blank sample, and 300 mg was accurately weighed and different concentrations of standard products were added, 6 were pretreated in parallel according to the pretreatment steps, and the recovery rate was calculated after the injection test. The concentrations of N-nitrosodimethylamine in the solution after spiking were 2 ng/mL, 10 ng/mL, and 100 ng/mL, respectively.
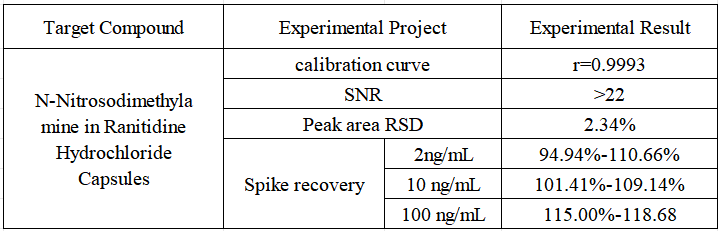
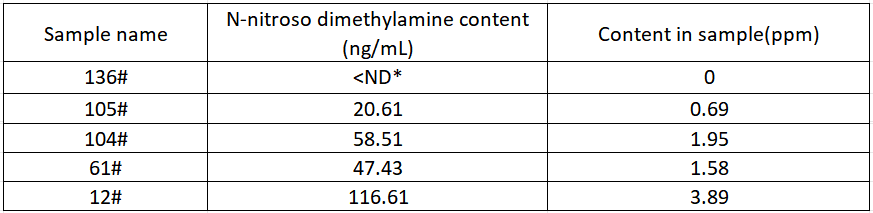
Sample test results
Ranitidine hydrochloride capsules according to the above sample preparation and instrument method, the samples are parallel 3 times, each parallel sample is injected 2 times consecutively, and the final average value is taken as the test result, and the test results are summarized as shown in Table 4 below.

Epilogue
In this paper, the content of N-nitrosodimethylamine in ranitidine hydrochloride capsules was detected by using the EXPEC 5210 LC-MS/MS to investigate the linearity, sensitivity, stability and spiked recovery of the method. This method is simple, sensitive and accurate, which can better meet the detection requirements of N-nitrosodimethylamine in ranitidine hydrochloride capsules.

