Cough and asthma drugs are commonly used in daily life. Regular Chinese patent medicines for treating asthma are generally slow to take effect. Unscrupulous traders often illegally add theophylline, prednisone acetate, diazepam and other chemicals to health products and Chinese patent medicines. Drugs are designed to achieve immediate results and induce consumers to buy them, thereby earning high profits. These illegal additives not only have good therapeutic effects, but also have serious adverse reactions , including nausea, nervousness, insomnia and even arrhythmia, induced epilepsy and death. However, patients who unknowingly take in large amounts of illegally added antitussive and asthmatic drugs without medical advice will cause serious harm to their bodies . Therefore, it is very important to monitor illegally added substances for relieving cough and asthma in health products and proprietary Chinese medicines.
The ingredients of health care products and proprietary Chinese medicines are complex, and criminals are becoming increasingly sophisticated in their illegal addition methods. Fast and accurate analysis methods are particularly important in the safety monitoring of health care products and proprietary Chinese medicines. The triple quadrupole tandem mass spectrometer can easily resolve complex matrix interferences and effectively deal with the complex component matrices of health care products and Chinese patent medicines. Its excellent sensitivity and stability can be well applied to the detection of illegal additions in health care products and Chinese patent medicines. Therefore, this application center established a fast, accurate, and sensitive method for analyzing the illegal addition of antitussive and antiasthmatic products in health care products and proprietary Chinese medicines based on a triple quadrupole tandem mass spectrometer.
Keywords: LC-MS/MS, food, health products, antitussive and asthma drugs, illegal addition
Experimental part
instrument
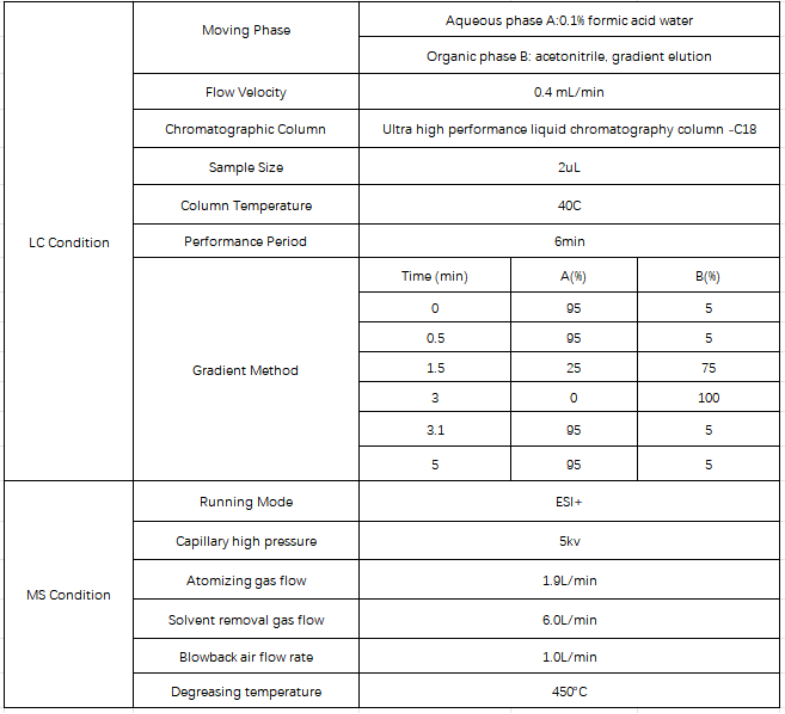
Table 1 Liquid chromatography tandem triple quadrupole mass spectrometer

Table 2 Detection parameters of liquid chromatography tandem triple quadrupole mass spectrometer
Reagents and standards
Reagents: chromatography grade acetonitrile (Merck), mass spectrometry grade formic acid (CNW);
Pure water: 18.2 MΩ·cm deionized water (25℃);
Standard products: theophylline, sulfamethoxazole, chlorpheniramine maleate, diphenhydramine hydrochloride, pentovirine citrate, phenproperine phosphate, prednisone acetate, and diazepam were purchased from Tianjin Alta.
Sample preparation
Break the 3g sample into pieces, extract it with 100mL methanol, pass it through the membrane, and put it on the machine.
Linearity and sensitivity
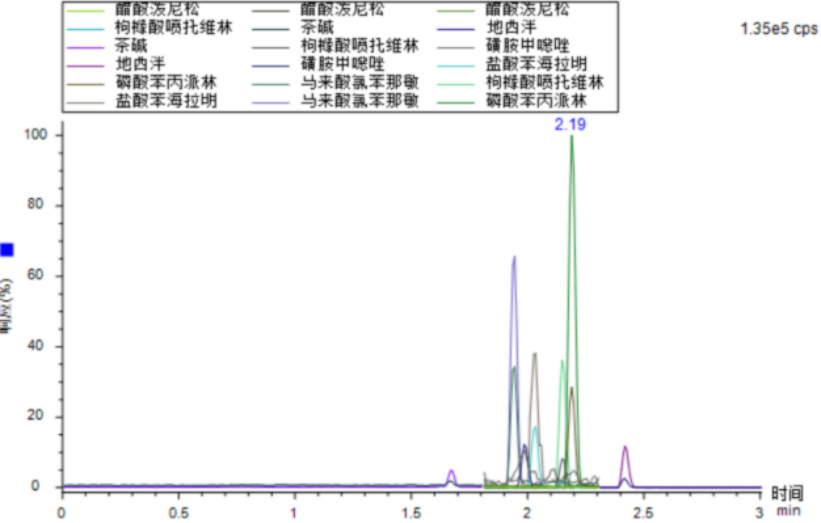
Figure 1 Typical spectrum of cough and asthma reference substance (100ng/mL)
Table 3 Linearity and signal-to-noise ratio of antitussive and antiasthmatic drugs
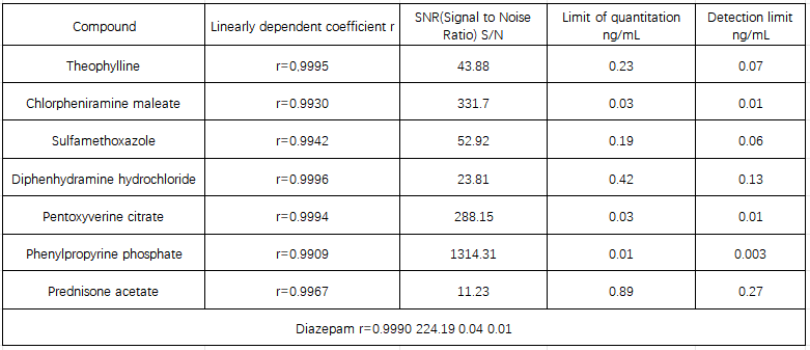
The signal-to-noise ratio is the signal-to-noise ratio at a concentration of 1ng/mL.
Cough and asthma substances have good linearity in the concentration range of 1-50ng/mL, and the correlation coefficients are all greater than 0.99; the quantification limit of each substance is between 0.01-0.89ng/mL; the detection limit is between 0.003-0.27ng/mL between.
Repeatability
Configure the 10ng/mL concentration reference substance and inject the sample 7 times continuously to check the repeatability of the response. The results are shown in the figure below.
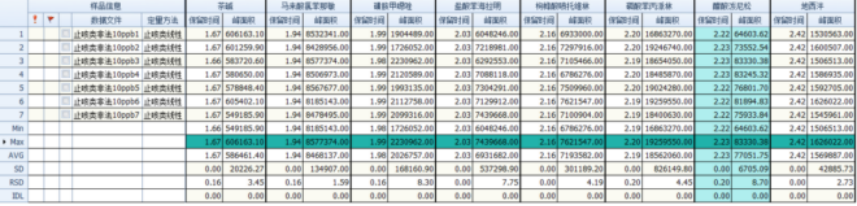
Figure 2 Precision of 10ng/mL antitussive and antiasthmatic compounds
10ng/mL repeatability: peak area RSD is between 1.59% and 8.70%, and retention time RSD is less than 0.20%.
Conclusion
This article established an analytical method using a triple quadrupole liquid mass spectrometer to determine the illegal addition of antitussive and antiasthmatic substances in Chinese patent medicines. This article also examined the linearity, repeatability, sensitivity, etc. of the method. The results show that: antitussive and antiasthmatic substances are The substances have good linearity in the concentration range of 1-50ng/mL, and the correlation coefficients are all greater than 0.99; the quantification limit of each substance is between 0.01-0.89ng/mL; the detection limit is between 0.003-0.27ng/mL. After seven consecutive injections, the RSD of each substance was within 10%, and the precision met the standard requirements. Therefore, this method can achieve rapid, accurate and sensitive quantitative determination of illegal additions of antitussive and antiasthmatic products to Chinese patent medicines.
appendix
Equipment and consumables solutions
1. EXPEC 5210 configuration details
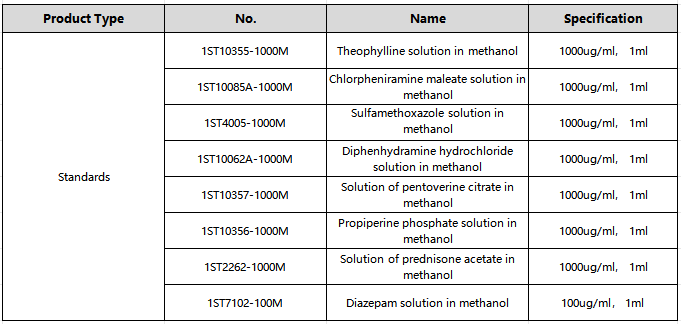
2. Standard products