Lithium carbonate has a wide range of uses and is an indispensable raw material in important industrial fields such as ceramics, energy, metallurgy, batteries, and medicine. The development of new energy sources has become a hot spot in the world, and lithium-ion batteries for power and energy storage have been developed rapidly. As the core raw material, lithium carbonate has a very broad market prospect. my country's lithium carbonate industry presents a high output of industrial-grade products, and relatively low output of battery-grade lithium carbonate, most of which rely on imports. Therefore, the technical research on purifying industrial-grade lithium carbonate into battery-grade lithium carbonate has become one of the research hotspots in the lithium battery industry. Compared with battery-grade lithium carbonate, a significant feature of industrial-grade lithium carbonate is its high impurity content. If you want to improve the purity of lithium carbonate, you must first understand the impurity content and types in industrial-grade lithium carbonate before you can use it according to the characteristics of each element. Propose corresponding solutions.
At the current stage, the determination standards for the content of impurity elements in lithium carbonate include spectrophotometry, electrode method, flame atomic absorption spectrometry, and inductively coupled plasma emission spectrometry (ICP-OES). Compared with other methods, inductively coupled plasma emission spectrometry has many advantages such as simple pretreatment process, wide linear range, low detection limit, and rapid simultaneous determination of multi-element content. The standards for determining the content of elemental impurities by ICP-OES include "Lithium Carbonate, Lithium Hydroxide Monohydrate, Lithium Chloride Chemical Analysis Methods Part 16: Calcium, Magnesium, Copper, Lead, Zinc, Nickel, Manganese, Cadmium, Aluminum Determination of Inductively Coupled Plasma Atomic Emission Spectrometry" (GB/T 11064.16-2013), "Determination of Na, K, Fe, Ca, Mg, B Content in Lithium Carbonate in Brine-Inductively Coupled Plasma Emission Spectrometry" (DB63/T 1297-2014). Referring to the above standards, the application center uses inductively coupled plasma emission spectrometry to accurately determine the contents of potassium, sodium, calcium, magnesium, aluminum, zinc and other elements in industrial grade lithium carbonate.
Keywords: lithium battery, lithium carbonate, ICP-OES
Experimental part
Instrument
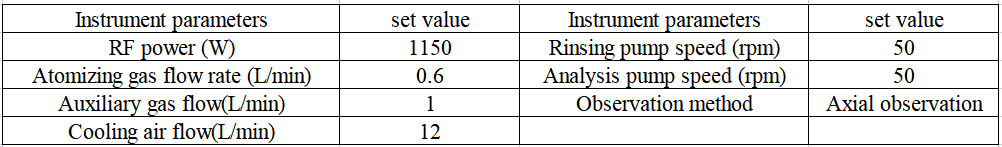
The configuration of EXPEC 6500 inductively coupled plasma emission spectrometer used in this paper is shown in Table 1.
Table 1 Instrument configuration

Table 2 Analysis and test parameters of inductively coupled plasma optical emission spectrometer
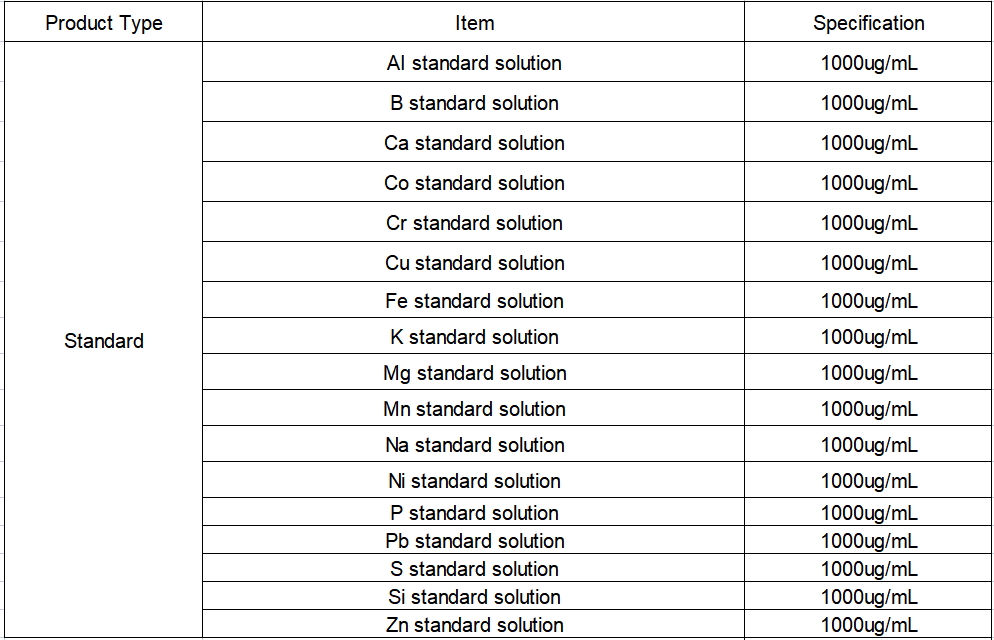
Reagents and Standard Solutions
Reagents: superior grade pure nitric acid, superior grade pure hydrochloric acid;
Ultrapure water: resistivity ≥ 18.2 MΩ cm (25°C), and other indicators meet the first-class standard of GB/T 6682;
High-purity argon: purity ≥ 99.999%; high-purity nitrogen: purity ≥ 99.999%;
Standard solution: Al, B, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, P, Pb, S, Si, Zn single element standard solution, ρ = 1000 μg/mL, National Nonferrous Metals Metal Institute.
Sample handling
Weigh the sample, add nitric acid, dissolve at low temperature, and then place it on a 120°C electric heating plate to reflux for 30 minutes. After cooling, transfer to a 50 mL plastic volumetric flask, and rinse the digestion cup with ultrapure water for 3 to 5 times, transfer the rinse solution to a 50 mL plastic volumetric flask, dilute to the mark with water, and shake well. Simultaneously prepare blank samples and parallel samples in the same way.
Test Results
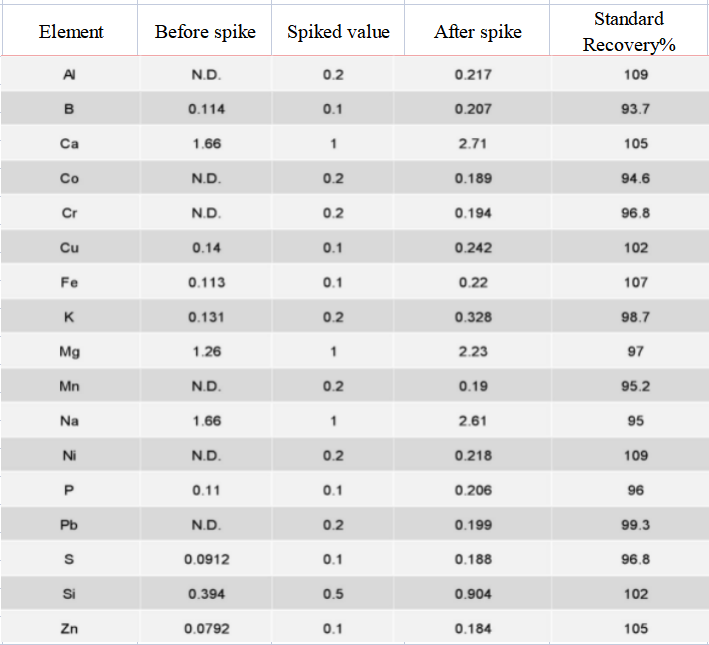
Spike recovery test
The standard addition test was carried out on the actual sample of lithium carbonate, and the precision test was carried out on 7 groups of standard spiked parallel samples. The recovery rate of each element spiked in the actual sample was 93.7% ~ 109%, indicating that the spiked test has a good recovery rate.
Table 4 Results of spiked test of analytes in samples (unit: mg/L)
The precision of 7 groups of parallel samples added with standard samples, the results show that the RSD of each parallel sample precision of each sample is less than 5%, which has good parallelism, which proves that the data is accurate and reliable.
Epilogue
In this paper, ICP-OES was used to determine the content of 17 elements such as aluminum, boron, calcium, cobalt, chromium and copper in industrial grade lithium carbonate. The linear coefficient of the standard curve of each analyte in the sample is greater than 0.99990, the precision test RSD of the analyte in the actual sample is less than 5.0%, and the recovery rate of each analyte in the actual sample is in the range of 93.7% ~ 109% , the element detection limit ranged from 0.01 to 0.97 mg/L. It shows that the precision and accuracy of the sample test are good, and the method can be effectively applied to the determination of 17 elements such as aluminum, boron, calcium, cobalt, chromium, and copper in industrial-grade lithium carbonate samples.
Appendix
Equipment and Consumables Solutions
1. Configuration details of EXPEC 6500D standard sampling system
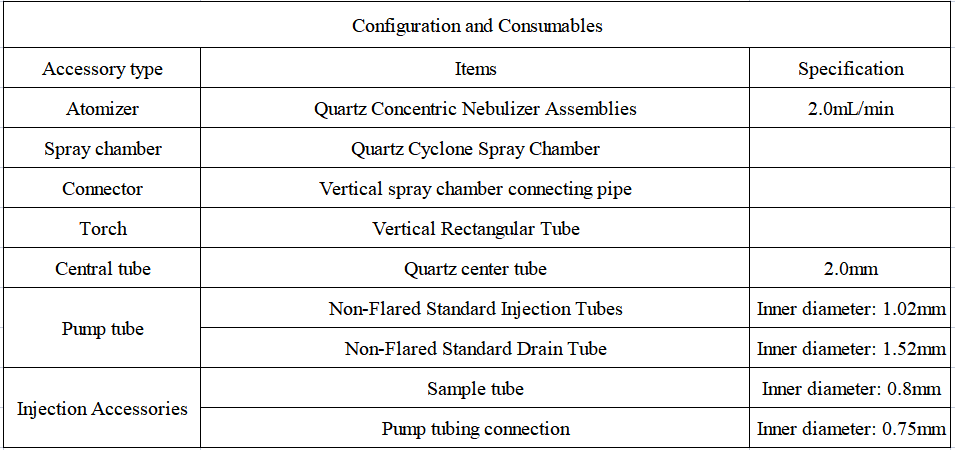
2. Reagents and Standards