Introduction
Plastics are one of the most important families of synthetic materials, with countless types used across modern industry. Compared with traditional materials like metal or wood, plastics are cheaper to produce, easier to process, and ideal for large-scale, automated manufacturing. As a result, the plastics sector has become a core part of China’s industrial foundation. In 2024 alone, national production reached 77.076 million tons, reflecting steady growth in light-industry development.
Identifying polymer types is essential for assessing material quality, ensuring product performance, and supporting environmental and regulatory work. A range of analytical techniques is used for polymer identification—such as infrared spectroscopy, thermal analysis, and pyrolysis-based methods. Pyrolysis analysis is one of the earliest and most established approaches; China released its first standard for polymer pyrolysis GC in 1986 (GB/T 7131-1986).
In 2024, the updated standard “Plastics — Identification of Polymers — Pyrolysis Gas Chromatography–Mass Spectrometry (SN/T 5689-2024)” introduced mass spectrometric detection and formally adopted Py/GC-MS for polymer classification. In Py/GC-MS, the sample is thermally decomposed in a pyrolyzer, and the resulting fragments are separated and identified by GC-MS. Chromatographic patterns, mass spectra, characteristic fragments, and retention indices together enable accurate polymer identification. The technique offers high sensitivity, requires only trace amounts of sample, and avoids the distortions that can arise from complex pretreatment.
In this study, the EXPEC 228 pyrolyzer and EXPEC 3750 GC-MS were used to analyze the pyrolysis profiles of eight common plastics. Retention indices were applied to support identification of characteristic compounds. The method is simple, fast, and highly reliable, making it a powerful tool for routine polymer monitoring.
Instruments and Reagents
Instruments:EXPEC 228 pyrolyzer;Gas Chromatography–Mass Spectrometer :EXPEC 3750 GC-MS, Hangzhou EXPEC Technology Co., Ltd.

Figure 1. EXPEC 228 pyrolyzer and EXPEC 3750 GC-MS Appearance
Reagents and Materials
High-density polyethylene (HDPE), polymethyl methacrylate (PMMA), polypropylene (PP), polyamide-6 (PA6), acrylonitrile-butadiene-styrene copolymer (ABS), polyethylene terephthalate (PET), polystyrene (PS), and polyvinyl chloride (PVC)..
Sample Preparation and Sampling
Using tools such as scissors, pliers, or a cryogenic mill, the samples were cut or ground into small fragments or powder with a particle size below 1 mm and thoroughly homogenized. Approximately 0.2 mg of the powder or small fragments was transferred into a sample cup using a micro-spatula or tweezers for analysis.
Analytical Reference Conditions
The reference operating conditions for the EXPEC 228 pyrolyzer and the EXPEC 3750 GC-MS system are listed in Table 1.
Table 1. Instrument Reference Conditions
| Py | Furnace Temperature | 600 ℃ |
| Interface temperature | 320 ℃ | |
| GC-MS | Inlet temperature | 310 ℃ |
| Injection mode | Split 100:1 | |
| Column | Rxi-5 MS, 30 m × 0.25 mm × 0.25 µm | |
| Carrier gas flow rate | 1.0 mL/min,constant flow | |
| Oven program | Initial 40 °C, hold 2 min; ramp at 20 °C/min to 310 °C, hold 20 min | |
| Transfer line temperature | 300 ℃ | |
| Ion source temperature | 280 ℃ | |
| Ionization mode | EI,70 eV | |
| Mass scan range | Scan(29 amu~600 amu) |
Results and Discussion
Eight samples were analyzed under the reference instrument conditions. The polymer types were identified based on the chromatograms, mass spectra, and retention indices of the target pyrolysis products.
Retention Index Determination
Following the instrument reference conditions, an n-alkane standard mixture (C8–C40) was introduced using the liquid injection module of the pyrolyzer. The total ion chromatogram (TIC) of the n-alkane mixture is shown in Figure 2.
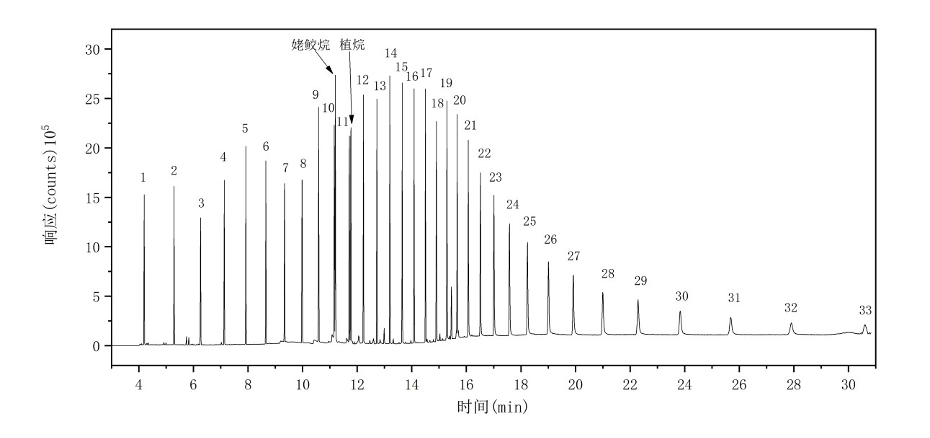
Figure 2. The total ion chromatogram (TIC) of the 19 PAEs.
Note:1-C8 ;2-C9 ;3-C10 ;4-C11 ;5-C12 ;6-C13 ;7-C14 ;8-C15 ;9-C16 ;10-C17 ;11-C18 ;12-C19 ;13-C20;14-C21 ;15-C22 ;16-C23 ;17-C24 ;18-C25 ;19-C26 ;20-C27 ;21-C28 ;22-C29 ;23-C30 ;24-C31 ;25-C32 ;26-C33 ;27-C34 ;28-C35 ;29-C36 ;30-C37 ;31-C38 ;32-C39 ;33-C40
The retention indices of the pyrolysis products from plastics were calculated using the retention times of the adjacent n-alkanes that bracket each target compound in the chromatogram. The retention index (RI) was obtained by logarithmic interpolation, according to the following formula:
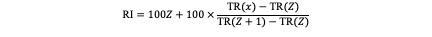
Where:
RI — retention index of the component;
Z — carbon number of the n-alkane eluting before the target compound;
TR(x) — retention time of the target compound;
TR(Z) — retention time of the n-alkane with carbon number Z;
TR(Z+1) — retention time of the n-alkane with carbon number Z+1.
Pyrolysis Analysis of Plastic Samples
(1) HDPE
The total ion chromatogram (TIC) of the HDPE sample is shown in Figure 3. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 2.
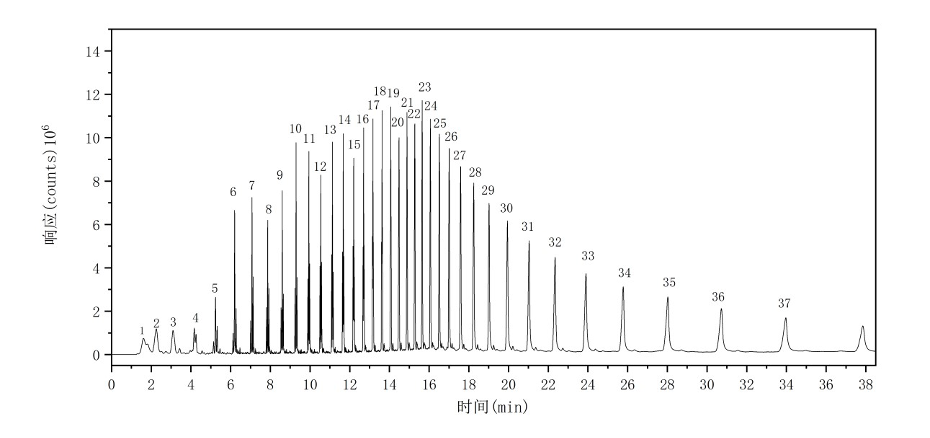
Figure 3: Total Ion Chromatogram of HDPE
Table 2: Major Pyrolysis Products of HDPE
| No. | Compound Name | Retention Time(min) | Retention Index |
| 1 | Propylene + Propane | 1.6057 | 300 |
| 2 | Hexene | 2.2577 | 584 |
| 3 | Heptene | 3.0602 | 690 |
| Heptane | 3.0948 | 700 | |
| 4 | 1,8-Octadiene | 4.0724 | 784 |
| Octene | 4.1689 | 792 | |
| Octane | 4.2578 | 800 | |
| 5 | 1,9-Nonadiene | 5.1455 | 884 |
| Nonene | 5.2376 | 893 | |
| Nonane | 5.3207 | 900 | |
| 6 | 1,10-Decadiene | 6.1262 | 984 |
| Decene | 6.2051 | 993 | |
| Decane | 6.2768 | 1000 | |
| 7 | 1,11-Undecadiene | 7.0032 | 1084 |
| Undecene | 7.0736 | 1093 | |
| Undecane | 7.1374 | 1100 | |
| 8 | 1,12-Dodecadiene | 7.8035 | 1184 |
| Dodecene | 7.8655 | 1193 | |
| Dodecane | 7.9251 | 1200 | |
| 9 | 1,13-Tridecadiene | 8.5462 | 1285 |
| Tridecene | 8.6034 | 1293 | |
| Tridecane | 8.6554 | 1300 | |
| 10 | 1,14-Tetradecadiene | 9.2386 | 1385 |
| Tetradecene | 9.2922 | 1394 | |
| Tetradecane | 9.3371 | 1400 | |
| 11 | 1,15-Pentadecadiene | 9.8893 | 1486 |
| Pentadecene | 9.9374 | 1487 | |
| Pentadecane | 9.9795 | 1500 | |
| 12 | 1,16-Hexadecadiene | 10.5038 | 1586 |
| Hexadecene | 10.5481 | 1594 | |
| Hexadecane | 10.5857 | 1600 | |
| 13 | 1,17-Heptadecadiene | 11.0865 | 1687 |
| Heptadecene | 11.1260 | 1694 | |
| Heptadecane | 11.1600 | 1700 | |
| 14 | 1,18-Octadecadiene | 11.6380 | 1787 |
| Octadecene | 11.6747 | 1795 | |
| Octadecane | 11.7051 | 1800 | |
| 15 | 1,19-Nonadecadiene | 12.1653 | 1888 |
| Nonadecene | 12.1978 | 1895 | |
| 16 | Nonadecane | 12.2251 | 1900 |
| 1,20-Eicosadiene | 12.6646 | 1988 | |
| Eicosene | 12.6943 | 1995 | |
| Eicosane | 12.7183 | 2000 | |
| 17 | Heneicosene | 13.1699 | 2091 |
| 18 | Docosene | 13.6261 | 2187 |
| 19 | Tricosene | 14.0639 | 2272 |
| 20 | Tetracosene | 14.4825 | 2356 |
| 21 | Pentacosene | 14.8861 | 2517 |
| 22 | Hexacosene | 15.2735 | 2592 |
| 23 | Heptacosene | 15.6511 | 2666 |
| 24 | Octacosene | 16.0620 | 2736 |
| 25 | Nonacosene | 16.5072 | 2806 |
| 26 | Triacontene | 17.0102 | 2993 |
| 27 | Hentriacontene | 17.5830 | 2957 |
| 28 | Dotriacontene | 18.2455 | 3044 |
| 29 | Tritriacontene | 19.0246 | 3142 |
| 30 | Tetratriacontene | 19.9432 | 3255 |
| 31 | Pentatriacontene | 21.0347 | 3385 |
| 32 | Hexatriacontene | 22.3427 | 3623 |
| 33 | Heptatriacontene | 23.9035 | 3712 |
| 34 | Octatriacontene | 25.7716 | 3801 |
| 35 | Nonatriacontene | 28.0208 | 3899 |
| 36 | Tetracontene | 30.7186 | 3977 |
| 37 | Henatetracontene | 33.9686 | 4096 |
(2) PMMA
The total ion chromatogram (TIC) of the PMMA sample is shown in Figure 4. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 3.
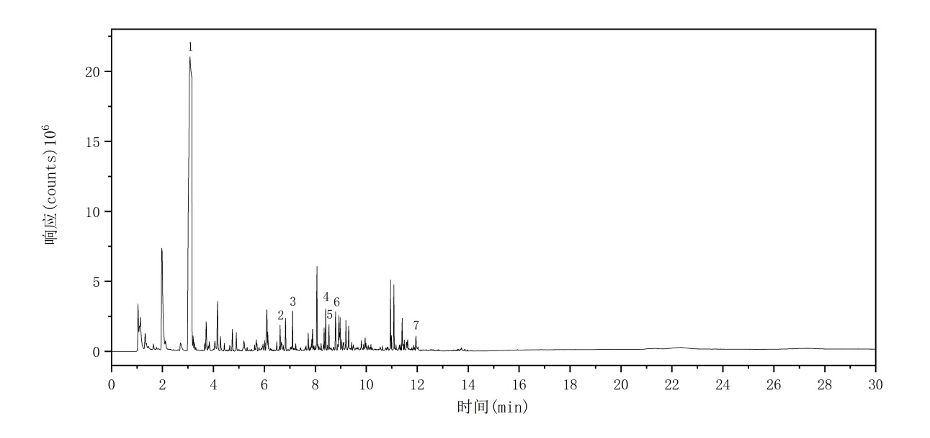
Figure 4: Total Ion Chromatogram of PMMA
Table 3: Major Pyrolysis Products of PMMA
| Compound Name | Retention Time (min) | Retention Index |
| Methyl isobutyrate | 3.0788 | 715 |
| Methyl 2,2,4-trimethyl-4-pentenoate | 6.6198 | 1041 |
| Methyl 2,4-dimethyl-2,4-pentadienoate | 7.1059 | 1097 |
| Methyl 2,2-dimethyl-4-methylene pentanoate | 8.4039 | 1266 |
| Methyl 2-isopropyl-4-methyl-2-pentenoate | 8.5325 | 1283 |
| Methyl 2,2,4-trimethyl-2-pentenoate | 8.7926 | 1320 |
| Methyl 4,6-dimethyl-2-heptenoate | 11.9480 | 1846 |
(3) PP
The total ion chromatogram (TIC) of the PP sample is shown in Figure 5. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 4.
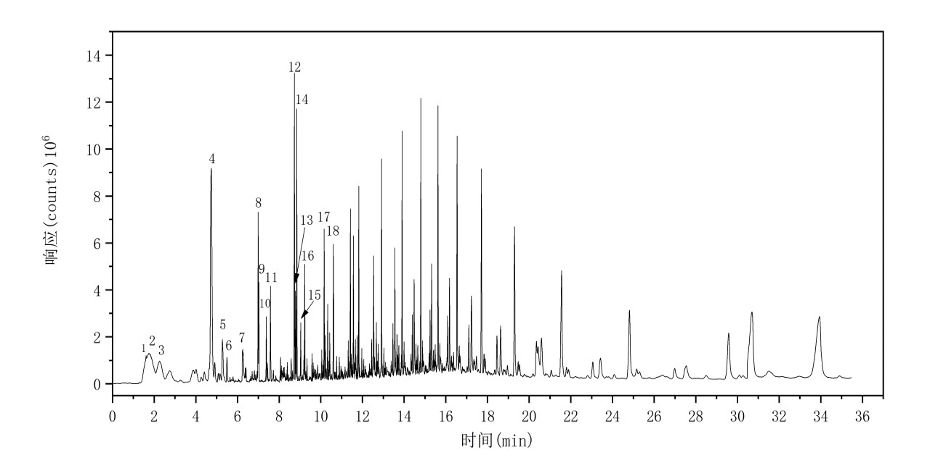
Figure 5 Total Ion Chromatogram of PP
Table 4 Major Pyrolysis Products of PP
| No. | Compound Name | Retention Time (min) | Retention Index |
| 1 | Propane | 1.6163 | 295 |
| 2 | n-Butane | 1.7563 | 500 |
| 3 | 2-Methylpropene | 2.2684 | 580 |
| 4 | 2,4-Dimethyl-1-pentene | 4.7344 | 849 |
| 5 | 2,4,6-Trimethyl-1-heptene | 5.2790 | 899 |
| 6 | 2,4,6-Trimethyl-1,6-heptadiene | 5.4938 | 921 |
| 7 | 4,6-Dimethyl-2-octene (internal) | 6.2447 | 998 |
| 8 | 2,4,6-Trimethyl-1-octene (internal) | 6.9932 | 1084 |
| 9 | 2,4,6-Trimethyl-1-octene (external) | 7.0249 | 1088 |
| 10 | 2,4,6,8-Tetramethyl-1-nonene (internal) | 7.3849 | 1132 |
| 11 | 2,4,6,8-Tetramethyl-1,8-nonadiene (internal) | 7.5837 | 1157 |
| 12 | 2,4,6,8-Tetramethyl-1-undecene (all-trans) | 8.7281 | 1311 |
| 13 | 2,4,6,8-Tetramethyl-1-undecene (anti-trans) | 8.7852 | 1319 |
| 14 | 2,4,6,8-Tetramethyl-1-undecene (syn-trans) | 8.8486 | 1328 |
| 15 | 2,4,6,8,10-Pentamethyl-1-undecene (all-trans) | 9.0338 | 1355 |
| 16 | 2,4,6,8,10-Pentamethyl-1-undecene (syn–syn–trans) | 9.2230 | 1383 |
| 17 | 2,4,6,8,10-Pentamethyl-1-undecene (all-cis) | 10.1610 | 1530 |
| 18 | 2,4,6,8,10,12-Hexamethyl-1,12-tridecadiene (trans–trans–trans) | 10.6064 | 1603 |
(4) PA6
The total ion chromatogram (TIC) of the PA6 sample is shown in Figure 6. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 5.
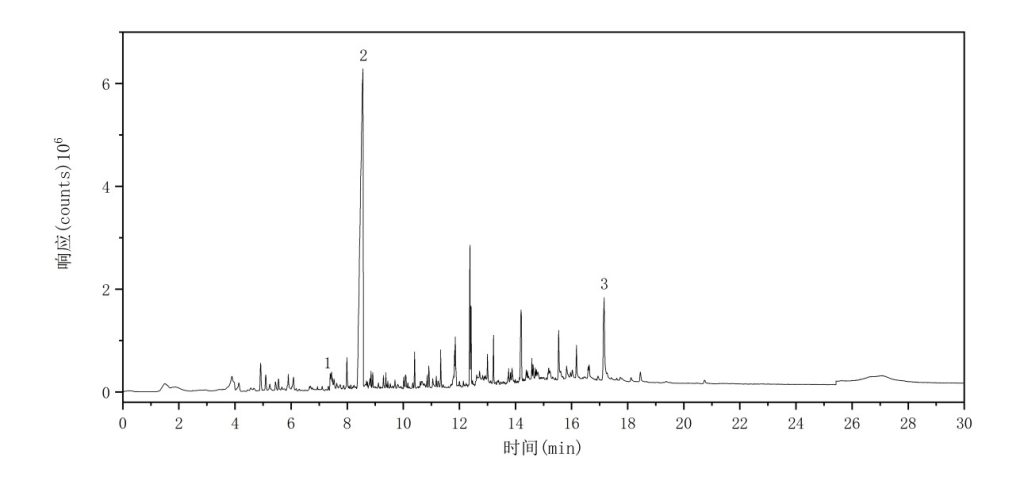
Figure 6 Total Ion Chromatogram of PA6
Table 5 Major Pyrolysis Products of PA6
| No. | Compound Name | Retention Time (min) | Retention Index |
| 1 | 6-Aminohexanenitrile | 7.3957 | 1133 |
| 2 | ε-Caprolactam | 8.5548 | 1286 |
| 3 | N-(5-Oxopentyl)-6-hexanamide | 17.1630 | 3026 |
(5) ABS
The total ion chromatogram (TIC) of the ABS sample is shown in Figure 7. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 6.
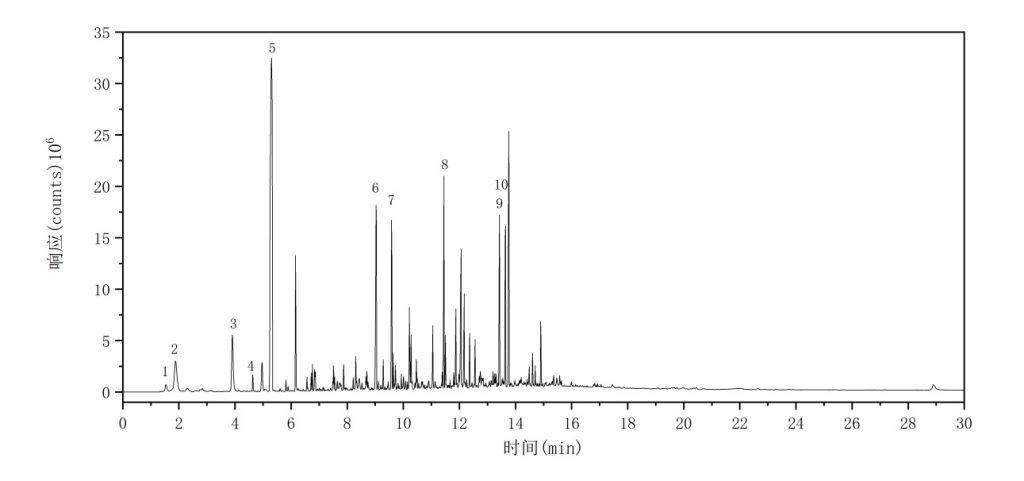
Figure 7 Total Ion Chromatogram of ABS
Table 6 Major Pyrolysis Products of ABS
| No. | Compound Name | Retention Time (min) | Retention Index |
| 1 | 1,3-Butadiene | 1.5402 | 395 |
| 2 | Acrylonitrile | 1.8803 | 560 |
| 3 | Toluene | 3.9039 | 766 |
| 4 | 4-Ethylcyclohexene | 4.6408 | 838 |
| 5 | Styrene | 5.3016 | 901 |
| 6 | 4-Methylstyrene | 9.5806 | 1438 |
| 7 | 4-Vinyltoluene | 10.2191 | 1539 |
| 8 | 1,3-Diphenylpropane | 11.4439 | 1749 |
| 9 | 2-Isopropyl-4,6-dimethylphenol | 13.4234 | 2150 |
| 10 | 4,6-Dimethyl-6-phenylheptanitrile | 13.6360 | 2197 |
(6) PET
The total ion chromatogram (TIC) of the PET sample is shown in Figure 8. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 7.
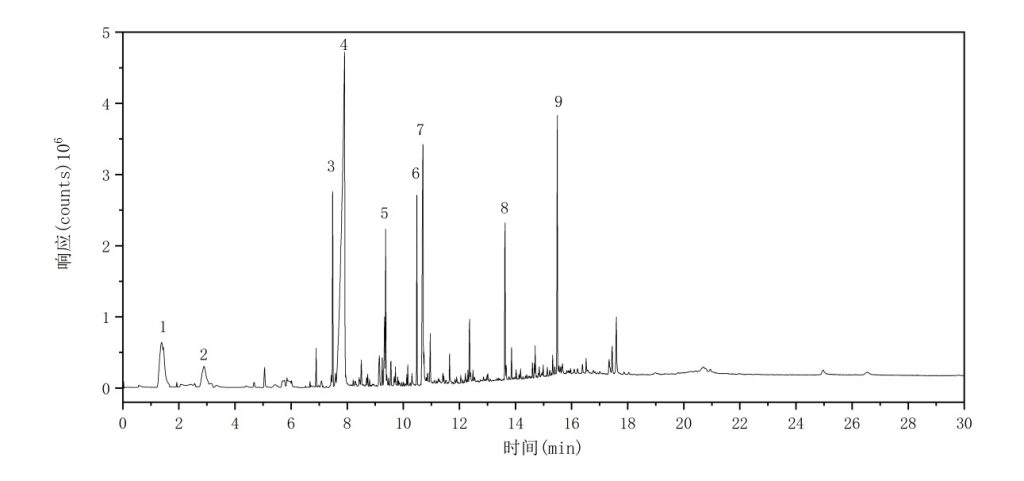
Figure 8 Total Ion Chromatogram of PET
Table 7 Major Pyrolysis Products of PET
| No. | Compound Name | Retention Time (min) | Retention Index |
| 1 | Acetaldehyde | 1.5518 | 408 |
| 2 | Benzene | 2.7717 | 658 |
| 3 | Ethyl benzoate | 7.4812 | 1144 |
| 4 | Benzoic acid | 7.9027 | 1198 |
| 5 | Biphenyl | 9.3704 | 1405 |
| 6 | Dimethyl terephthalate | 10.4829 | 1583 |
| 7 | 4-Formylmethyl benzoate | 10.6925 | 1618 |
| 8 | Diethyl terephthalate | 13.6226 | 2194 |
| 9 | Bis(2-hydroxyethyl) terephthalate | 15.4880 | 2654 |
(7) PS
The total ion chromatogram (TIC) of the PS sample is shown in Figure 9. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 8.
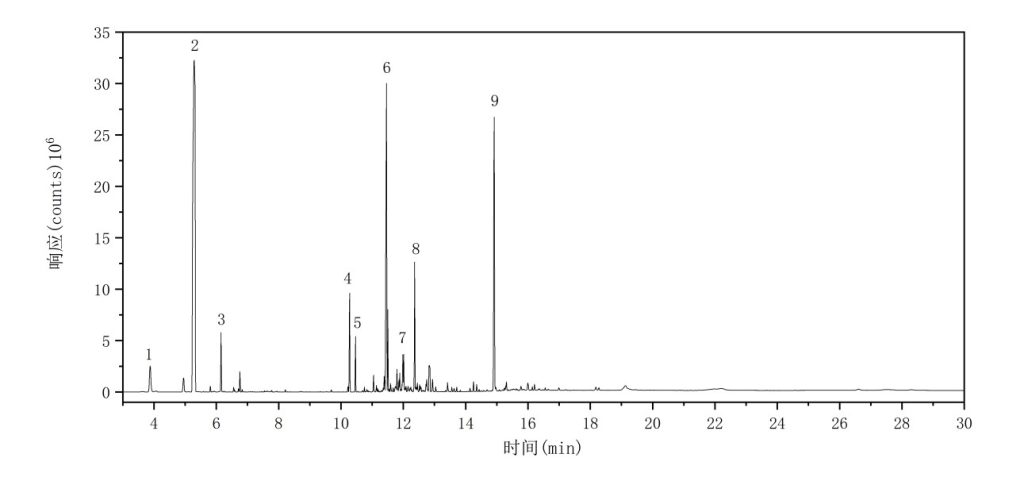
Figure 9 Total Ion Chromatogram of PS
Table 8 Major Pyrolysis Products of PS
| No. | Compound Name | Retention Time (min) | Retention Index |
| 1 | Toluene | 3.8690 | 767 |
| 2 | Ethylbenzene | 5.2916 | 900 |
| 3 | α-Methylstyrene | 6.1526 | 988 |
| 4 | 1,2-Diphenylethane | 10.2738 | 1548 |
| 5 | 1,3-Diphenylpropane | 10.4639 | 1579 |
| 6 | 1,3-Diphenyl-3-butene | 11.4560 | 1753 |
| 7 | 1,4-Diphenyl-1-butene | 11.9833 | 1852 |
| 8 | 2,5-Diphenyl-1,5-pentadiene | 12.3631 | 1927 |
| 9 | 1,3,5-Triphenyl-5-pentene (styrene trimer)) | 14.9124 | 2502 |
(8) PVC
The total ion chromatogram (TIC) of the PVC sample is shown in Figure 10. The major characteristic pyrolysis products and their corresponding retention indices are summarized in Table 9.
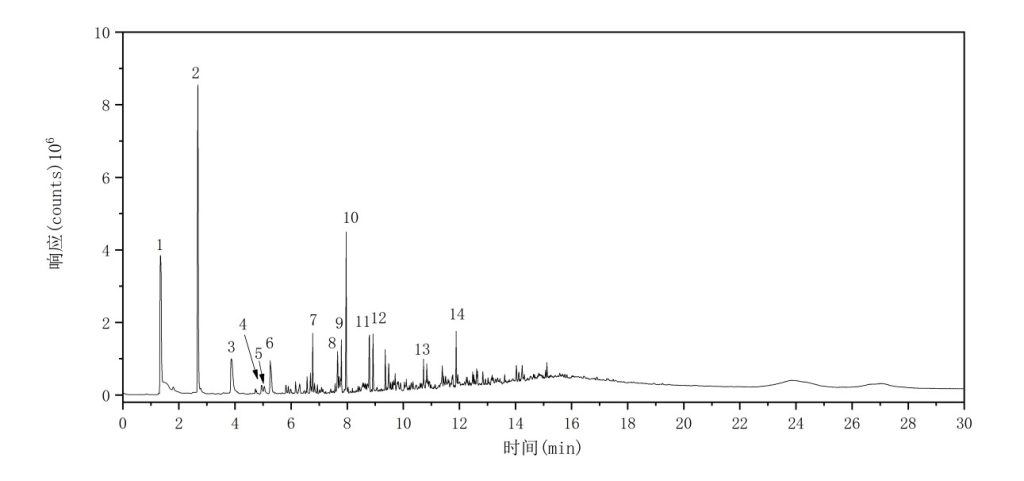
Figure 10 TIC of PVC
Table 9 Major Pyrolysis Products of PVC
| No. | Compound Name | Retention Time (min) | Retention Index |
| 1 | Hydrochloric acid | 1.3452 | 245 |
| 2 | Benzene | 2.6752 | 651 |
| 3 | Toluene | 3.8692 | 767 |
| 4 | Ethylbenzene | 4.9460 | 868 |
| 5 | Xylenes (o-, m-, or p-xylene) | 5.0443 | 877 |
| 6 | Styrene | 5.2623 | 897 |
| 7 | Ethylbenzene | 6.7705 | 1058 |
| 8 | 1-Methylnaphthalene | 7.6559 | 1166 |
| 9 | 3-Methylnaphthalene | 7.7905 | 1183 |
| 10 | Naphthalene | 7.9600 | 1205 |
| 11 | 2-Methylnaphthalene | 8.7871 | 1319 |
| 12 | 1-Ethylnaphthalene | 8.9208 | 1338 |
| 13 | Fluorene | 10.7212 | 1622 |
| 14 | Anthracene | 11.8844 | 1833 |
Conclusion
Using the EXPEC 228 pyrolyzer coupled with the EXPEC 3750 GC-MS system, eight types of plastics were analyzed through their characteristic pyrolysis products, and the polymer types were successfully identified. The method fully meets the analytical requirements of the standard “Plastics — Identification of Polymers — Pyrolysis Gas Chromatography–Mass Spectrometry (SN/T 5689-2024)”. It features simple operation, minimal sample consumption, no need for reference standards, and high accuracy. This approach is therefore well-suited for routine monitoring and identification of plastic polymers.

