In the production process of stainless steel, the oxidation resistance and corrosion resistance of stainless steel are mainly improved by controlling the chromium content in stainless steel. Therefore, it is very important and necessary to establish a reliable and accurate analysis method for chromium content in stainless steel.
When using ICP-OES for chromium analysis, the pre-treatment often uses an electric heating plate to heat it and then digest it for sampling and testing. However, for high-carbon steel electric heating plate digestion, some carbon residues will remain, and the digestion is not thorough. The chromium element will react with carbon to form chromium carbide precipitation, affecting the test results. At this time, it is necessary to add strong oxidizing acids, such as perchloric acid and sulfuric acid. However, it has been reported in the literature that when perchloric acid is used to decompose the sample, about 10% of the chromium will volatilize in the form of CrOCl 3 , and the recovery rate is low. If concentrated sulfuric acid is used to increase the boiling point, although it can be completely digested, its high viscosity will affect the introduction of the test solution. Therefore, neither is the optimal solution.
This article introduces the pretreatment method of selecting super microwave digestion and using only aqua regia for digestion when measuring chromium in steel, and compares the test results with the method of heating with a hot plate.
1. Experimental Procedure
laboratory apparatus
EXPEC 790S Super Microwave Digestion System

EXPEC 6500 ICP-OES

Experimental samples
High speed tool steel, YSB S 11483-2009
Sample preparation
Accurately weigh 0.1000 g of the sample to be tested into a digestion tube, add 4 mL of hydrochloric acid and 1 mL of nitric acid in sequence, and mix well. Place the digestion tube into the digestion tube rack, transfer it to the super microwave platform, pre-pressurize it to 4 MPa, digest it according to the super microwave heating program, cool it down, and then dilute it to 100 mL with deionized water. At the same time, do a blank experiment.
The digestion program was set as follows:
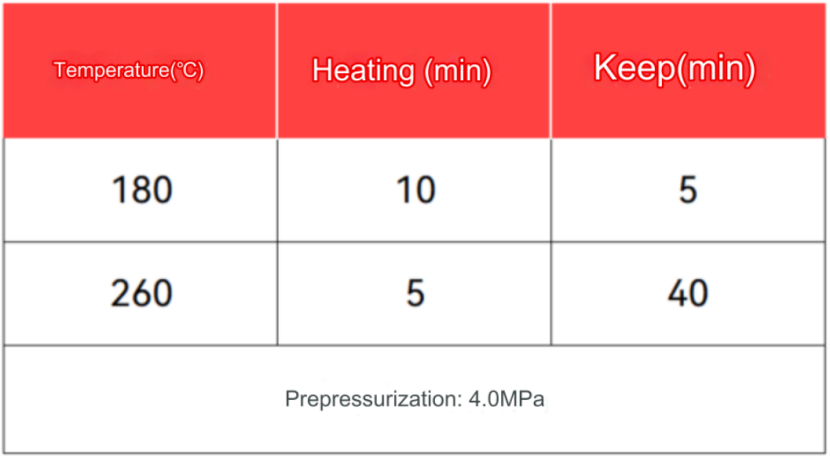
Accurately weigh 0.1000g of the sample to be tested into a polytetrafluoroethylene beaker, add 4mL hydrochloric acid and 1mL nitric acid in turn, and mix well. Heat on a hot plate at 180℃ for 1h to digest. After cooling, dilute to 100mL with deionized water, and perform a blank test at the same time.
Digestion effect
Carbon-coated cathode sample digestion


Before digestion
After digestion
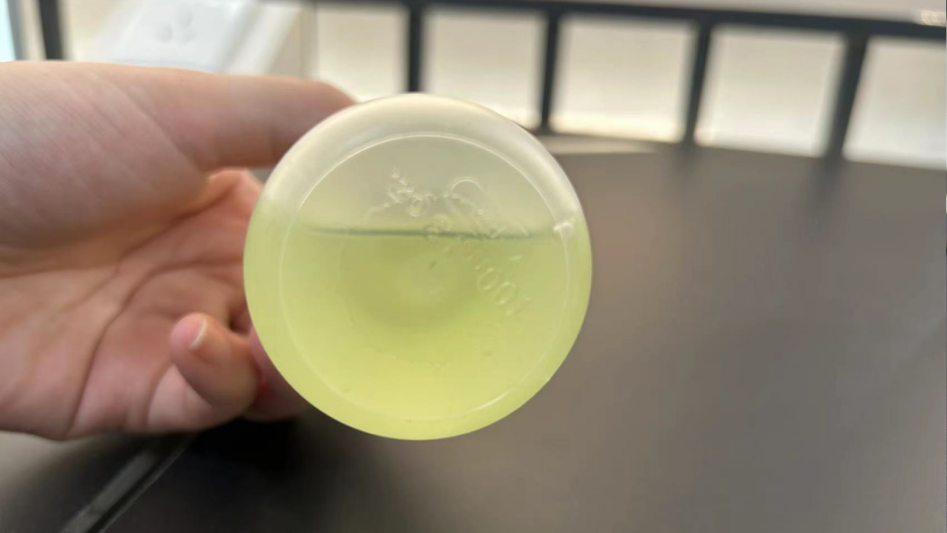

After super microwave digestion
After electric heating plate digestion
It can be clearly seen from the picture that when using aqua regia, there is still a large amount of black carbon precipitation after digestion on the hot plate.
ICP-OES test results
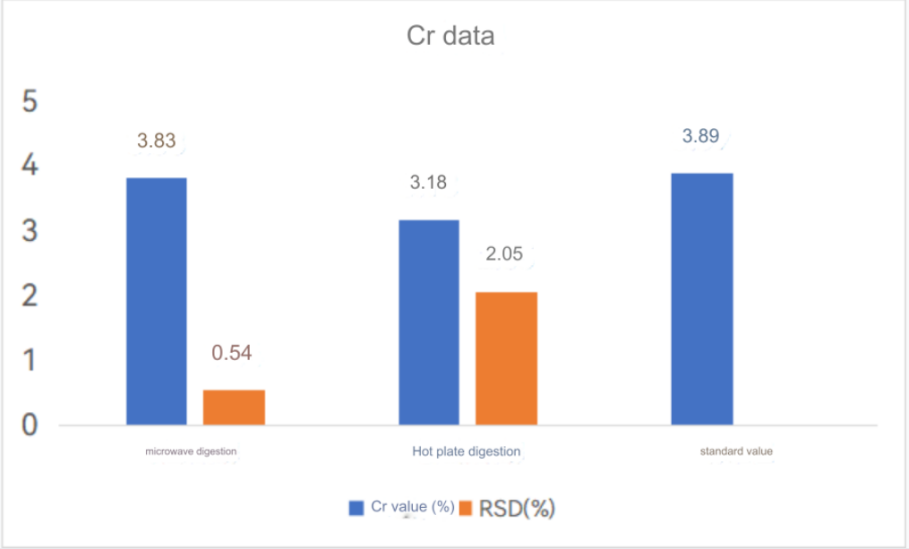
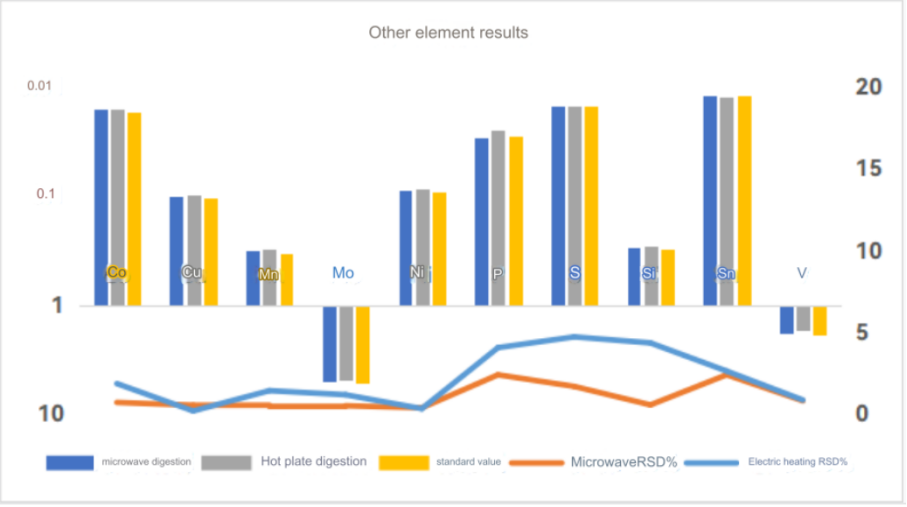
Chromium standard curve
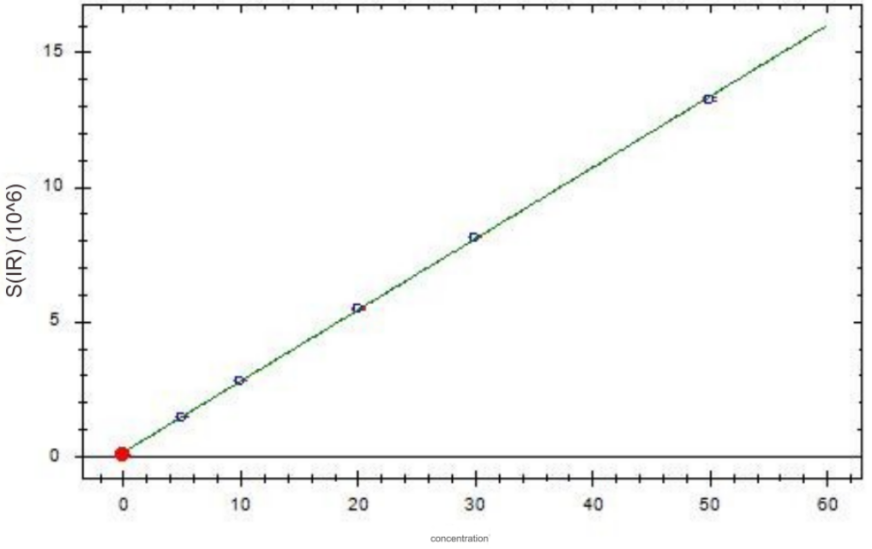
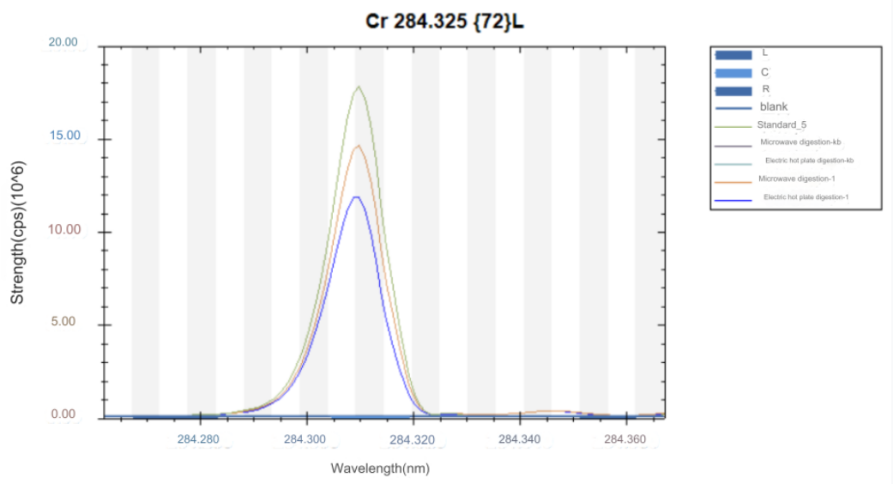
2. Experimental conclusion
High carbon steel was digested by electric heating plate and microwave digestion. The results of the two digestion methods were compared. Super microwave can completely oxidize and digest carbides, while electric heating plate digestion has some carbon residues and the digestion is not thorough.
The test data of using aqua regia to digest high carbon steel in super microwave showed that the test value of chromium element was consistent with the standard value. Other elements were also almost within the standard value range, so this method can solve the difficulty of chromium element analysis in high carbon steel.

